Page 41 - Read Online
P. 41
Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38 Page 5 of 54
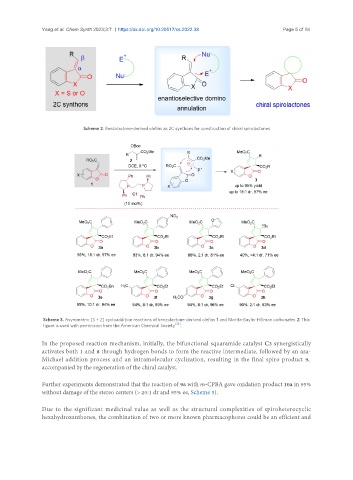
Scheme 2. Benzolactone-derived olefins as 2C synthons for construction of chiral spirolactones.
Scheme 3. Asymmetric [3 + 2] cycloaddition reactions of benzolactone-derived olefins 1 and Morita-Baylis-Hillman carbonates 2. This
figure is used with permission from the American Chemical Society [32] .
In the proposed reaction mechanism, initially, the bifunctional squaramide catalyst C3 synergistically
activates both 1 and 8 through hydrogen bonds to form the reactive intermediate, followed by an aza-
Michael addition process and an intramolecular cyclization, resulting in the final spiro product 9,
accompanied by the regeneration of the chiral catalyst.
Further experiments demonstrated that the reaction of 9a with m-CPBA gave oxidation product 10a in 95%
without damage of the stereo centers (> 20:1 dr and 95% ee, Scheme 5).
Due to the significant medicinal value as well as the structural complexities of spiroheterocyclic
hexahydroxanthones, the combination of two or more known pharmacophores could be an efficient and