Page 24 - Read Online
P. 24
Wu et al. Chem Synth 2023;3:6 https://dx.doi.org/10.20517/cs.2022.42 Page 5 of 17
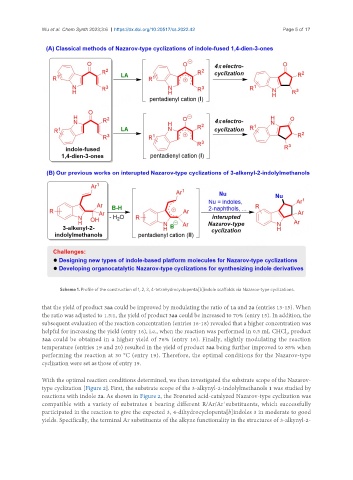
Scheme 1. Profile of the construction of 1, 2, 3, 4-tetrahydrocyclopenta[b]indole scaffolds via Nazarov-type cyclizations.
that the yield of product 3aa could be improved by modulating the ratio of 1a and 2a (entries 13-15). When
the ratio was adjusted to 1.5:1, the yield of product 3aa could be increased to 70% (entry 15). In addition, the
subsequent evaluation of the reaction concentration (entries 16-18) revealed that a higher concentration was
helpful for increasing the yield (entry 16), i.e., when the reaction was performed in 0.5 mL CHCl , product
3
3aa could be obtained in a higher yield of 76% (entry 16). Finally, slightly modulating the reaction
temperature (entries 19 and 20) resulted in the yield of product 3aa being further improved to 85% when
performing the reaction at 30 °C (entry 19). Therefore, the optimal conditions for the Nazarov-type
cyclization were set as those of entry 19.
With the optimal reaction conditions determined, we then investigated the substrate scope of the Nazarov-
type cyclization [Figure 2]. First, the substrate scope of the 3-alkynyl-2-indolylmethanols 1 was studied by
reactions with indole 2a. As shown in Figure 2, the Brønsted acid-catalyzed Nazarov-type cyclization was
1
compatible with a variety of substrates 1 bearing different R/Ar/Ar substituents, which successfully
participated in the reaction to give the expected 3, 4-dihydrocyclopenta[b]indoles 3 in moderate to good
yields. Specifically, the terminal Ar substituents of the alkyne functionality in the structures of 3-alkynyl-2-