Page 22 - Read Online
P. 22
Wu et al. Chem Synth 2023;3:6 https://dx.doi.org/10.20517/cs.2022.42 Page 3 of 17
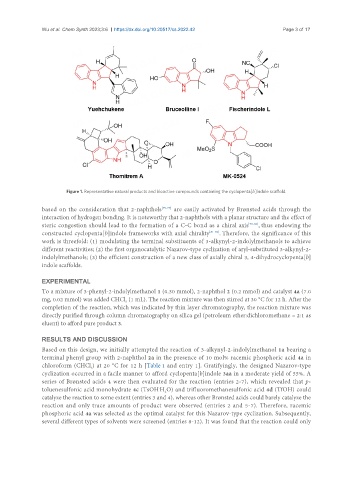
Figure 1. Representative natural products and bioactive compounds containing the cyclopenta[b]indole scaffold.
based on the consideration that 2-naphthols [75-77] are easily activated by Brønsted acids through the
interaction of hydrogen bonding. It is noteworthy that 2-naphthols with a planar structure and the effect of
steric congestion should lead to the formation of a C-C bond as a chiral axis [78-80] , thus endowing the
constructed cyclopenta[b]indole frameworks with axial chirality [81-89] . Therefore, the significance of this
work is threefold: (1) modulating the terminal substituents of 3-alkynyl-2-indolylmethanols to achieve
different reactivities; (2) the first organocatalytic Nazarov-type cyclization of aryl-substituted 3-alkynyl-2-
indolylmethanols; (3) the efficient construction of a new class of axially chiral 3, 4-dihydrocyclopenta[b]
indole scaffolds.
EXPERIMENTAL
To a mixture of 3-phenyl-2-indolylmethanol 1 (0.30 mmol), 2-naphthol 2 (0.2 mmol) and catalyst 4a (7.0
mg, 0.02 mmol) was added CHCl (1 mL). The reaction mixture was then stirred at 30 °C for 12 h. After the
3
completion of the reaction, which was indicated by thin layer chromatography, the reaction mixture was
directly purified through column chromatography on silica gel (petroleum ether:dichloromethane = 2:1 as
eluent) to afford pure product 3.
RESULTS AND DISCUSSION
Based on this design, we initially attempted the reaction of 3-alkynyl-2-indolylmethanol 1a bearing a
terminal phenyl group with 2-naphthol 2a in the presence of 10 mol% racemic phosphoric acid 4a in
chloroform (CHCl ) at 20 °C for 12 h [Table 1 and entry 1]. Gratifyingly, the designed Nazarov-type
3
cyclization occurred in a facile manner to afford cyclopenta[b]indole 3aa in a moderate yield of 55%. A
series of Brønsted acids 4 were then evaluated for the reaction (entries 2-7), which revealed that p-
.
toluenesulfonic acid monohydrate 4c (TsOHH O) and trifluoromethanesulfonic acid 4d (TfOH) could
2
catalyze the reaction to some extent (entries 3 and 4), whereas other Brønsted acids could barely catalyze the
reaction and only trace amounts of product were observed (entries 2 and 5-7). Therefore, racemic
phosphoric acid 4a was selected as the optimal catalyst for this Nazarov-type cyclization. Subsequently,
several different types of solvents were screened (entries 8-12). It was found that the reaction could only