Page 25 - Read Online
P. 25
Page 6 of 17 Wu et al. Chem Synth 2023;3:6 https://dx.doi.org/10.20517/cs.2022.42
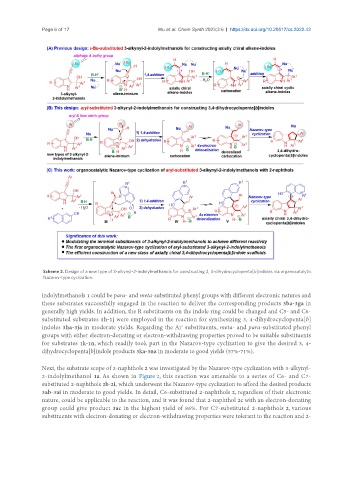
Scheme 2. Design of a new type of 3-alkynyl-2-indolylmethanols for constructing 3, 4-dihydrocyclopenta[b]indoles via organocatalytic
Nazarov-type cyclization.
indolylmethanols 1 could be para- and meta-substituted phenyl groups with different electronic natures and
these substrates successfully engaged in the reaction to deliver the corresponding products 3ba-3ga in
generally high yields. In addition, the R substituents on the indole ring could be changed and C5- and C6-
substituted substrates 1h-1j were employed in the reaction for synthesizing 3, 4-dihydrocyclopenta[b]
indoles 3ha-3ja in moderate yields. Regarding the Ar substituents, meta- and para-substituted phenyl
1
groups with either electron-donating or electron-withdrawing properties proved to be suitable substituents
for substrates 1k-1n, which readily took part in the Nazarov-type cyclization to give the desired 3, 4-
dihydrocyclopenta[b]indole products 3ka-3na in moderate to good yields (57%-71%).
Next, the substrate scope of 2-naphthols 2 was investigated by the Nazarov-type cyclization with 3-alkynyl-
2-indolylmethanol 1a. As shown in Figure 2, this reaction was amenable to a series of C6- and C7-
substituted 2-naphthols 2b-2i, which underwent the Nazarov-type cyclization to afford the desired products
3ab-3ai in moderate to good yields. In detail, C6-substituted 2-naphthols 2, regardless of their electronic
nature, could be applicable to the reaction, and it was found that 2-naphthol 2c with an electron-donating
group could give product 3ac in the highest yield of 86%. For C7-substituted 2-naphthols 2, various
substituents with electron-donating or electron-withdrawing properties were tolerant to the reaction and 2-