Page 29 - Read Online
P. 29
Page 10 of 17 Wu et al. Chem Synth 2023;3:6 https://dx.doi.org/10.20517/cs.2022.42
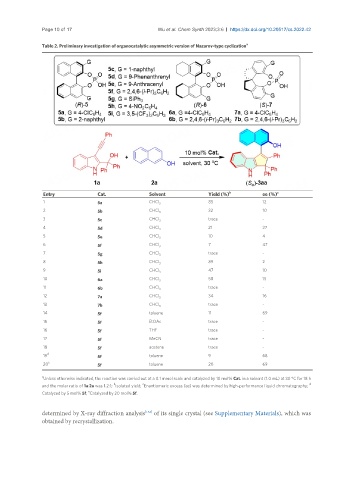
Table 2. Preliminary investigation of organocatalytic asymmetric version of Nazarov-type cyclization a
Entry Cat. Solvent Yield (%) b ee (%) c
1 5a CHCl 3 85 12
2 5b CHCl 3 32 10
3 5c CHCl 3 trace -
4 5d CHCl 3 21 27
5 5e CHCl 3 10 4
6 5f CHCl 3 7 47
7 5g CHCl 3 trace -
8 5h CHCl 3 89 2
9 5i CHCl 3 47 10
10 6a CHCl 3 58 15
11 6b CHCl 3 trace -
12 7a CHCl 3 34 16
13 7b CHCl 3 trace -
14 5f toluene 11 69
15 5f EtOAc trace -
16 5f THF trace -
17 5f MeCN trace -
18 5f acetone trace -
d
19 5f toluene 9 68
e
20 5f toluene 20 69
a
Unless otherwise indicated, the reaction was carried out at a 0.1 mmol scale and catalyzed by 10 mol% Cat. in a solvent (1.0 mL) at 30 °C for 18 h
c
b
and the molar ratio of 1a:2a was 1.2:1; Isolated yield; Enantiomeric excess (ee) was determined by high-performance liquid chromatography; d
e
Catalyzed by 5 mol% 5f; Catalyzed by 20 mol% 5f.
determined by X-ray diffraction analysis of its single crystal (see Supplementary Materials), which was
[152]
obtained by recrystallization.