Page 15 - Read Online
P. 15
Ji et al. Chem Synth 2022;2:17 https://dx.doi.org/10.20517/cs.2022.27 Page 7 of 11
a a
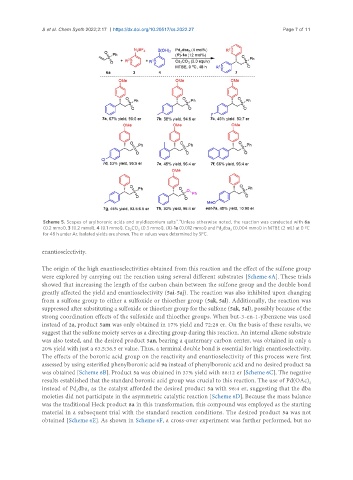
Scheme 5. Scopes of arylboronic acids and aryldiazonium salts . Unless otherwise noted, the reaction was conducted with 6a
(0.2 mmol), 3 (0.2 mmol), 4 (0.1 mmol), Cs CO (0.3 mmol), (R)-1a (0.012 mmol) and Pd dba (0.004 mmol) in MTBE (2 mL) at 0 ºC
2 3 2 3
for 48 h under Ar. Isolated yields are shown. The er values were determined by SFC.
enantioselectivity.
The origin of the high enantioselectivities obtained from this reaction and the effect of the sulfone group
were explored by carrying out the reaction using several different substrates [Scheme 6A]. These trials
showed that increasing the length of the carbon chain between the sulfone group and the double bond
greatly affected the yield and enantioselectivity (5ai-5aj). The reaction was also inhibited upon changing
from a sulfone group to either a sulfoxide or thioether group (5ak, 5al). Additionally, the reaction was
suppressed after substituting a sulfoxide or thioether group for the sulfone (5ak, 5al), possibly because of the
strong coordination effects of the sulfoxide and thioether groups. When but-3-en-1-ylbenzene was used
instead of 2a, product 5am was only obtained in 17% yield and 72:28 er. On the basis of these results, we
suggest that the sulfone moiety serves as a directing group during this reaction. An internal alkene substrate
was also tested, and the desired product 5an, bearing a quaternary carbon center, was obtained in only a
20% yield with just a 63.5:36.5 er value. Thus, a terminal double bond is essential for high enantioselectivity.
The effects of the boronic acid group on the reactivity and enantioselectivity of this process were first
assessed by using esterified phenylboronic acid 9a instead of phenylboronic acid and no desired product 5a
was obtained [Scheme 6B]. Product 5a was obtained in 37% yield with 88:12 er [Scheme 6C]. The negative
results established that the standard boronic acid group was crucial to this reaction. The use of Pd(OAc)
2
instead of Pd dba as the catalyst afforded the desired product 5a with 96:4 er, suggesting that the dba
3
2
moieties did not participate in the asymmetric catalytic reaction [Scheme 6D]. Because the mass balance
was the traditional Heck product 8a in this transformation, this compound was employed as the starting
material in a subsequent trial with the standard reaction conditions. The desired product 5a was not
obtained [Scheme 6E]. As shown in Scheme 6F, a cross-over experiment was further performed, but no