Page 10 - Read Online
P. 10
Page 2 of 11 Ji et al. Chem Synth 2022;2:17 https://dx.doi.org/10.20517/cs.2022.27
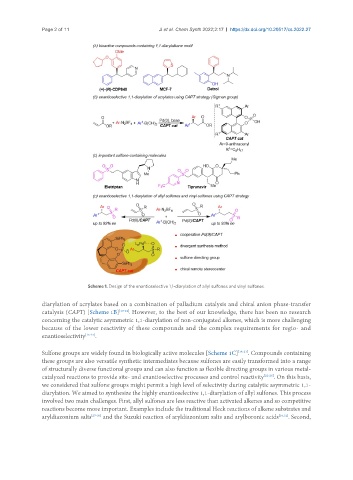
Scheme 1. Design of the enantioselective 1,1-diarylation of allyl sulfones and vinyl sulfones.
diarylation of acrylates based on a combination of palladium catalysis and chiral anion phase-transfer
catalysis (CAPT) [Scheme 1B] [10-14] . However, to the best of our knowledge, there has been no research
concerning the catalytic asymmetric 1,1-diarylation of non-conjugated alkenes, which is more challenging
because of the lower reactivity of these compounds and the complex requirements for regio- and
enantioselectivity [15-17] .
Sulfone groups are widely found in biologically active molecules [Scheme 1C] [18-21] . Compounds containing
these groups are also versatile synthetic intermediates because sulfones are easily transformed into a range
of structurally diverse functional groups and can also function as flexible directing groups in various metal-
catalyzed reactions to provide site- and enantioselective processes and control reactivity [22-26] . On this basis,
we considered that sulfone groups might permit a high level of selectivity during catalytic asymmetric 1,1-
diarylation. We aimed to synthesize the highly enantioselective 1,1-diarylation of allyl sulfones. This process
involved two main challenges. First, allyl sulfones are less reactive than activated alkenes and so competitive
reactions become more important. Examples include the traditional Heck reactions of alkene substrates and
aryldiazonium salts [27-30] and the Suzuki reaction of aryldiazonium salts and arylboronic acids [16,31] . Second,