Page 11 - Read Online
P. 11
Ji et al. Chem Synth 2022;2:17 https://dx.doi.org/10.20517/cs.2022.27 Page 3 of 11
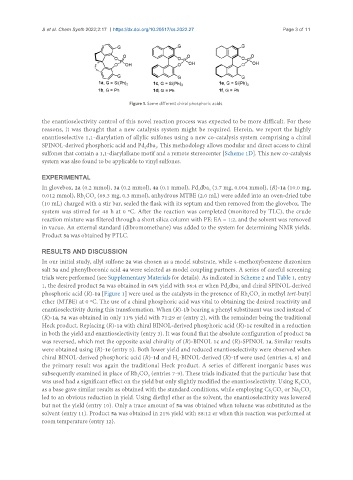
Figure 1. Some different chiral phosphoric acids.
the enantioselectivity control of this novel reaction process was expected to be more difficult. For these
reasons, it was thought that a new catalysis system might be required. Herein, we report the highly
enantioselective 1,1-diarylation of allylic sulfones using a new co-catalysis system comprising a chiral
SPINOL-derived phosphoric acid and Pd dba . This methodology allows modular and direct access to chiral
2
3
sulfones that contain a 1,1-diarylalkane motif and a remote stereocenter [Scheme 1D]. This new co-catalysis
system was also found to be applicable to vinyl sulfones.
EXPERIMENTAL
In glovebox, 2a (0.2 mmol), 3a (0.2 mmol), 4a (0.1 mmol), Pd dba (3.7 mg, 0.004 mmol), (R)-1a (10.0 mg,
2
3
0.012 mmol), Rb CO (69.3 mg, 0.3 mmol), anhydrous MTBE (2.0 mL) were added into an oven-dried tube
2
3
(10 mL) charged with a stir bar, sealed the flask with its septum and then removed from the glovebox. The
system was stirred for 48 h at 0 ºC. After the reaction was completed (monitored by TLC), the crude
reaction mixture was filtered through a short silica column with PE: EA = 1:2, and the solvent was removed
in vacuo. An external standard (dibromomethane) was added to the system for determining NMR yields.
Product 5a was obtained by PTLC.
RESULTS AND DISCUSSION
In our initial study, allyl sulfone 2a was chosen as a model substrate, while 4-methoxybenzene diazonium
salt 3a and phenylboronic acid 4a were selected as model coupling partners. A series of careful screening
trials were performed (see Supplementary Materials for details). As indicated in Scheme 2 and Table 1, entry
1, the desired product 5a was obtained in 64% yield with 96:4 er when Pd dba and chiral SPINOL-derived
3
2
phosphoric acid (R)-1a [Figure 1] were used as the catalysts in the presence of Rb CO in methyl tert-butyl
3
2
ether (MTBE) at 0 ºC. The use of a chiral phosphoric acid was vital to obtaining the desired reactivity and
enantioselectivity during this transformation. When (R)-1b bearing a phenyl substituent was used instead of
(R)-1a, 5a was obtained in only 11% yield with 71:29 er (entry 2), with the remainder being the traditional
Heck product. Replacing (R)-1a with chiral BINOL-derived phosphoric acid (R)-1c resulted in a reduction
in both the yield and enantioselectivity (entry 3). It was found that the absolute configuration of product 5a
was reversed, which met the opposite axial chirality of (R)-BINOL 1c and (R)-SPINOL 1a. Similar results
were obtained using (R)-1e (entry 5). Both lower yield and reduced enantioselectivity were observed when
chiral BINOL-derived phosphoric acid (R)-1d and H -BINOL-derived (R)-1f were used (entries 4, 6) and
8
the primary result was again the traditional Heck product. A series of different inorganic bases was
subsequently examined in place of Rb CO (entries 7-9). These trials indicated that the particular base that
2
3
was used had a significant effect on the yield but only slightly modified the enantioselectivity. Using K CO
3
2
as a base gave similar results as obtained with the standard conditions, while employing Cs CO or Na CO
3
3
2
2
led to an obvious reduction in yield. Using diethyl ether as the solvent, the enantioselectivity was lowered
but not the yield (entry 10). Only a trace amount of 5a was obtained when toluene was substituted as the
solvent (entry 11). Product 5a was obtained in 21% yield with 88:12 er when this reaction was performed at
room temperature (entry 12).