Page 132 - Read Online
P. 132
Xu et al. Chem Synth 2023;3:17 https://dx.doi.org/10.20517/cs.2022.35 Page 5 of 10
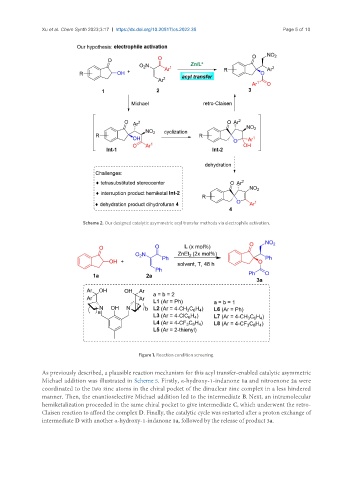
Scheme 2. Our designed catalytic asymmetric acyl transfer methods via electrophile activation.
Figure 1. Reaction condition screening.
As previously described, a plausible reaction mechanism for this acyl transfer-enabled catalytic asymmetric
Michael addition was illustrated in Scheme 5. Firstly, α-hydroxy-1-indanone 1a and nitroenone 2a were
coordinated to the two zinc atoms in the chiral pocket of the dinuclear zinc complex in a less hindered
manner. Then, the enantioselective Michael addition led to the intermediate B. Next, an intramolecular
hemiketalization proceeded in the same chiral pocket to give intermediate C, which underwent the retro-
Claisen reaction to afford the complex D. Finally, the catalytic cycle was restarted after a proton exchange of
intermediate D with another α-hydroxy-1-indanone 1a, followed by the release of product 3a.