Page 131 - Read Online
P. 131
Page 4 of 10 Xu et al. Chem Synth 2023;3:17 https://dx.doi.org/10.20517/cs.2022.35
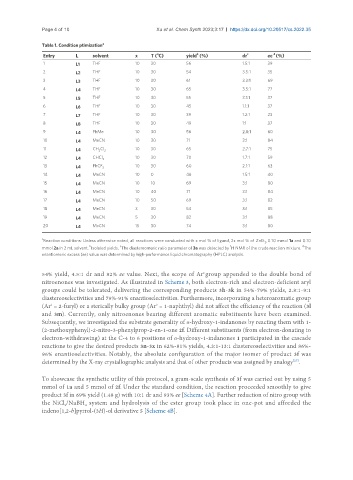
Table 1. Condition ptimization a
o
Entry L solvent x T ( C) yield (%) dr c ee (%)
d
b
1 L1 THF 10 30 56 1.5:1 39
2 L2 THF 10 30 54 3.5:1 35
3 L3 THF 10 30 61 3.3:1 69
4 L4 THF 10 30 65 3.5:1 77
5 L5 THF 10 30 55 3.1:1 37
6 L6 THF 10 30 45 1.1:1 37
7 L7 THF 10 30 39 1.2:1 23
8 L8 THF 10 30 49 1:1 37
9 L4 PhMe 10 30 56 2.8:1 60
10 L4 MeCN 10 30 71 3:1 84
11 L4 CH Cl 2 10 30 65 2.7:1 75
2
12 L4 CHCl 3 10 30 70 1.7:1 59
13 L4 PhCF 3 10 30 60 2.1:1 63
14 L4 MeCN 10 0 46 1.5:1 40
15 L4 MeCN 10 10 69 3:1 80
16 L4 MeCN 10 40 71 3:1 84
17 L4 MeCN 10 50 69 3:1 82
18 L4 MeCN 3 30 54 3:1 85
19 L4 MeCN 5 30 82 3:1 88
20 L4 MeCN 15 30 74 3:1 80
a
Reaction conditions: Unless otherwise noted, all reactions were conducted with x mol % of ligand, 2x mol % of ZnEt , 0.10 mmol 1a and 0.10
2
1
b
c
d
mmol 2a in 2 mL solvent. Isolated yields. The diastereomeric ratio parameter of 3a was detected by H NMR of the crude reaction mixture. The
enantiomeric excess (ee) value was determined by high-performance liquid chromatography (HPLC) analysis.
54% yield, 4.5:1 dr and 82% ee value. Next, the scope of Ar group appended to the double bond of
2
nitroenones was investigated. As illustrated in Scheme 3, both electron-rich and electron-deficient aryl
groups could be tolerated, delivering the corresponding products 3h-3k in 54%-79% yields, 2.8:1-9:1
diastereoselectivities and 79%-91% enantioselectivities. Furthermore, incorporating a heteroaromatic group
2
2
(Ar = 2-furyl) or a sterically bulky group (Ar = 1-naphthyl) did not affect the efficiency of the reaction (3l
and 3m). Currently, only nitroenones bearing different aromatic substituents have been examined.
Subsequently, we investigated the substrate generality of α-hydroxy-1-indanones by reacting them with 1-
(2-methoxyphenyl)-2-nitro-3-phenylprop-2-en-1-one 2f. Different substituents (from electron-donating to
electron-withdrawing) at the C-4 to 6 positions of α-hydroxy-1-indanones 1 participated in the cascade
reactions to give the desired products 3n-3x in 62%-81% yields, 4.3:1-13:1 diastereoselectivities and 86%-
96% enantioselectivities. Notably, the absolute configuration of the major isomer of product 3f was
determined by the X-ray crystallographic analysis and that of other products was assigned by analogy .
[27]
To showcase the synthetic utility of this protocol, a gram-scale synthesis of 3f was carried out by using 5
mmol of 1a and 5 mmol of 2f. Under the standard condition, the reaction proceeded smoothly to give
product 3f in 69% yield (1.48 g) with 10:1 dr and 93% ee [Scheme 4A]. Further reduction of nitro group with
the NiCl /NaBH system and hydrolysis of the ester group took place in one-pot and afforded the
2
4
indeno[1,2-b]pyrrol-(3H)-ol derivative 5 [Scheme 4B].