Page 16 - Read Online
P. 16
Page 228 Venkatesh et al. Cancer Drug Resist 2021;4:223-32 I http://dx.doi.org/10.20517/cdr.2020.84
A B
C
D
E
F
G
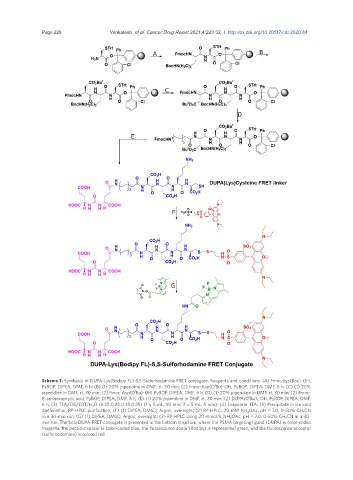
Scheme 1. Synthesis of DUPA-Lys(Bodipy FL)-S,S-Sulforhodamine FRET conjugate. Reagents and conditions: (A) Fmoc-Lys(Boc)-OH,
t
PyBOP, DIPEA, DMF, 6 h; (B) (1) 20% piperidine in DMF, rt, 30 min; (2) Fmoc-Asp(O Bu)-OH, PyBOP, DIPEA, DMF, 6 h; (C) (1) 20%
t
piperidine in DMF, rt, 30 min; (2) Fmoc-Asp(O Bu)-OH, PyBOP, DIPEA, DMF, 6 h; (D) (1) 20% piperidine in DMF, rt, 30 min; (2) Fmoc-
t
8-aminocaprylic acid, PyBOP, DIPEA, DMF, 6 h; (E) (1) 20% piperidine in DMF, rt, 30 min; (2) DUPA(O Bu) 3 -OH, PyBOP, DIPEA, DMF,
6 h; (3) TFA/TIS/EDT/H 2 O (9.25:0.25:0.25:0.25) (1 × 5 mL, 30 min; 2 × 5 mL, 5 min); (4) Evaporate TFA; (5) Precipitate in ice cold
diethylether, RP-HPLC purification; (F) (1) DIPEA, DMSO, Argon, overnight; (2) RP-HPLC, 20 mM NH 4 OAc, pH = 7.0, 0-50% CH 3 CN
in a 30-min run; (G) (1) DIPEA, DMSO, Argon, overnight; (2) RP-HPLC using 20 mmol/L NH 4 OAc, pH = 7.0, 0-50% CH 3 CN in a 30-
min run. The final DUPA-FRET conjugate is presented in the bottom structure, where the PSMA targeting ligand (DUPA) is color-coded
magenta, the peptidic spacer is color-coded blue, the fluorescence donor (Bodipy) is represented green, and the fluorescence acceptor
(sulforhodamine) is colored red