Page 21 - Read Online
P. 21
Page 12 of 15 Chen et al. Cancer Drug Resist 2024;7:9 https://dx.doi.org/10.20517/cdr.2023.151
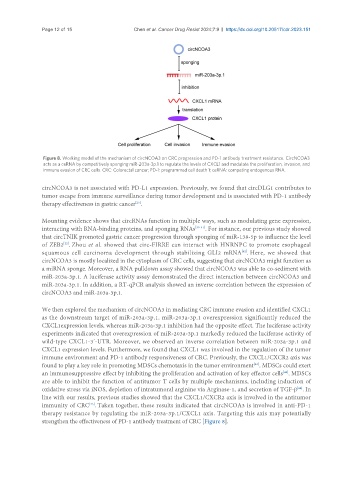
Figure 8. Working model of the mechanism of circNCOA3 on CRC progression and PD-1 antibody treatment resistance. CircNCOA3
acts as a ceRNA by competitively sponging miR-203a-3p.1 to regulate the levels of CXCL1 and modulate the proliferation, invasion, and
immune evasion of CRC cells. CRC: Colorectal cancer; PD-1: programmed cell death 1; ceRNA: competing endogenous RNA.
circNCOA3 is not associated with PD-L1 expression. Previously, we found that circDLG1 contributes to
tumor escape from immune surveillance during tumor development and is associated with PD-1 antibody
therapy effectiveness in gastric cancer .
[25]
Mounting evidence shows that circRNAs function in multiple ways, such as modulating gene expression,
interacting with RNA-binding proteins, and sponging RNAs [39-41] . For instance, our previous study showed
that circTNIK promoted gastric cancer progression through sponging of miR-138-5p to influence the level
of ZEB2 . Zhou et al. showed that circ-FIRRE can interact with HNRNPC to promote esophageal
[22]
[42]
squamous cell carcinoma development through stabilizing GLI2 mRNA . Here, we showed that
circNCOA3 is mostly localized in the cytoplasm of CRC cells, suggesting that circNCOA3 might function as
a miRNA sponge. Moreover, a RNA pulldown assay showed that circNCOA3 was able to co-sediment with
miR-203a-3p.1. A luciferase activity assay demonstrated the direct interaction between circNCOA3 and
miR-203a-3p.1. In addition, a RT-qPCR analysis showed an inverse correlation between the expression of
circNCOA3 and miR-203a-3p.1.
We then explored the mechanism of circNCOA3 in mediating CRC immune evasion and identified CXCL1
as the downstream target of miR-203a-3p.1. miR-203a-3p.1 overexpression significantly reduced the
CXCL1expression levels, whereas miR-203a-3p.1 inhibition had the opposite effect. The luciferase activity
experiments indicated that overexpression of miR-203a-3p.1 markedly reduced the luciferase activity of
wild-type CXCL1-3’-UTR. Moreover, we observed an inverse correlation between miR-203a-3p.1 and
CXCL1 expression levels. Furthermore, we found that CXCL1 was involved in the regulation of the tumor
immune environment and PD-1 antibody responsiveness of CRC. Previously, the CXCL1/CXCR2 axis was
found to play a key role in promoting MDSCs chemotaxis in the tumor environment . MDSCs could exert
[43]
an immunosuppressive effect by inhibiting the proliferation and activation of key effector cells . MDSCs
[44]
are able to inhibit the function of antitumor T cells by multiple mechanisms, including induction of
[44]
oxidative stress via iNOS, depletion of intratumoral arginine via Arginase-1, and secretion of TGF-β . In
line with our results, previous studies showed that the CXCL1/CXCR2 axis is involved in the antitumor
immunity of CRC . Taken together, these results indicated that circNCOA3 is involved in anti-PD-1
[45]
therapy resistance by regulating the miR-203a-3p.1/CXCL1 axis. Targeting this axis may potentially
strengthen the effectiveness of PD-1 antibody treatment of CRC [Figure 8].