Page 42 - Read Online
P. 42
Sooi et al. Cancer Drug Resist 2023;6:656-73 https://dx.doi.org/10.20517/cdr.2023.48 Page 658
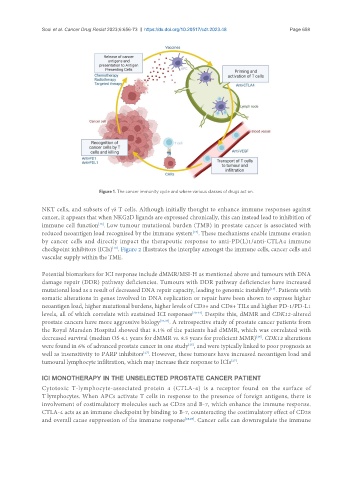
Figure 1. The cancer immunity cycle and where various classes of drugs act on.
NKT cells, and subsets of γδ T cells. Although initially thought to enhance immune responses against
cancer, it appears that when NKG2D ligands are expressed chronically, this can instead lead to inhibition of
[16]
immune cell function . Low tumour mutational burden (TMB) in prostate cancer is associated with
reduced neoantigen load recognised by the immune system . These mechanisms enable immune evasion
[17]
by cancer cells and directly impact the therapeutic response to anti-PD(L)1/anti-CTLA4 immune
checkpoint inhibitors (ICIs) . Figure 2 illustrates the interplay amongst the immune cells, cancer cells and
[18]
vascular supply within the TME.
Potential biomarkers for ICI response include dMMR/MSI-H as mentioned above and tumours with DNA
damage repair (DDR) pathway deficiencies. Tumours with DDR pathway deficiencies have increased
[19]
mutational load as a result of decreased DNA repair capacity, leading to genomic instability . Patients with
somatic alterations in genes involved in DNA replication or repair have been shown to express higher
neoantigen load, higher mutational burdens, higher levels of CD3+ and CD8+ TILs and higher PD-1/PD-L1
levels, all of which correlate with sustained ICI responses [20-24] . Despite this, dMMR and CDK12-altered
prostate cancers have more aggressive biology [25,26] . A retrospective study of prostate cancer patients from
the Royal Marsden Hospital showed that 8.1% of the patients had dMMR, which was correlated with
decreased survival (median OS 4.1 years for dMMR vs. 8.5 years for proficient MMR) . CDK12 alterations
[26]
[25]
were found in 6% of advanced prostate cancer in one study , and were typically linked to poor prognosis as
well as insensitivity to PARP inhibitors . However, these tumours have increased neoantigen load and
[27]
tumoural lymphocyte infiltration, which may increase their response to ICIs .
[27]
ICI MONOTHERAPY IN THE UNSELECTED PROSTATE CANCER PATIENT
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is a receptor found on the surface of
T lymphocytes. When APCs activate T cells in response to the presence of foreign antigens, there is
involvement of costimulatory molecules such as CD28 and B-7, which enhance the immune response.
CTLA-4 acts as an immune checkpoint by binding to B-7, counteracting the costimulatory effect of CD28
and overall cause suppression of the immune response [28,29] . Cancer cells can downregulate the immune