Page 45 - Read Online
P. 45
Page 661 Sooi et al. Cancer Drug Resist 2023;6:656-73 https://dx.doi.org/10.20517/cdr.2023.48
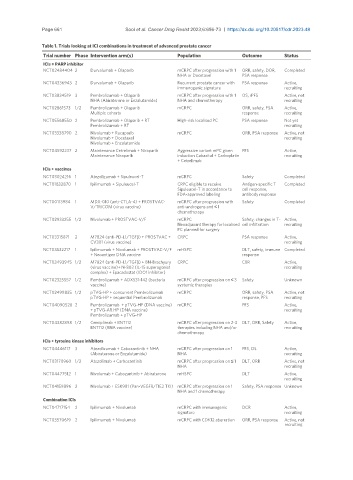
Table 1. Trials looking at ICI combinations in treatment of advanced prostate cancer
Trial number Phase Intervention arm(s) Population Outcome Status
ICIs + PARP inhibitor
NCT02484404 2 Durvalumab + Olaparib mCRPC after progression with 1 ORR, safety, DOR, Completed
NHA or Docetaxel PSA response
NCT04336943 2 Durvalumab + Olaparib Recurrent prostate cancer with PSA response Active,
immunogenic signature recruiting
NCT03834519 3 Pembrolizumab + Olaparib mCRPC after progression with 1 OS, rPFS Active, not
NHA (Abiraterone or Enzalutamide) NHA and chemotherapy recruiting
NCT02861573 1/2 Pembrolizumab + Olaparib mCRPC ORR, safety, PSA Active,
Multiple cohorts response recruiting
NCT05568550 2 Pembrolizumab + Olaparib + RT High-risk localised PC PSA response Not yet
Pembrolizumab + RT recruiting
NCT03338790 2 Nivolumab + Rucaparib mCRPC ORR, PSA response Active, not
Nivolumab + Docetaxel recruiting
Nivolumab + Enzalutamide
NCT04592237 2 Maintenance Cetrelimab + Niraparib Aggressive variant mPC given PFS Active,
Maintenance Niraparib induction Cabazital + Carboplatin recruiting
+ Cetrelimab
ICIs + vaccines
NCT03024216 1 Atezolizumab + Sipuleucel-T mCRPC Safety Completed
NCT01832870 1 Ipilimumab + Sipuleucel-T CRPC eligible to receive Antigen-specific T Completed
Sipuleucel-T in accordance to cell response,
FDA-approved labeling antibody response
NCT00113984 1 MDX-010 (anti-CTLA-4) + PROSTVAC- mCRPC after progression with Safety Completed
V/TRICOM (virus vaccine) anti-androgens and ≤ 1
chemotherapy
NCT02933255 1/2 Nivolumab + PROSTVAC-V/F mCRPC Safety, changes in T- Active,
Neoadjuvant therapy for localised cell infiltration recruiting
PC planned for surgery
NCT03315871 2 M7824 (anti-PD-L1/TGFβ) + PROSTVAC + CRPC PSA response Active,
CV301 (virus vaccine) recruiting
NCT03532217 1 Ipilimumab + Nivolumab + PROSTVAC-V/F mHSPC DLT, safety, immune Completed
+ Neoantigen DNA vaccine response
NCT03493945 1/2 M7824 (anti-PD-L1/TGFβ) + BN-Brachyury CRPC CBR Active,
(virus vaccine)+ N-803 (IL-15 superagonist recruiting
complex) + Epacadostat (IDO1 inhibitor)
NCT02325557 1/2 Pembrolizumab + ADXS31-142 (bacteria mCRPC after progression on ≤ 3 Safety Unknown
vaccine) systemic therapies
NCT02499835 1/2 pTVG-HP + concurrent Pembrolizumab mCRPC ORR, safety, PSA Active, not
pTVG-HP + sequential Pembrolizumab response, PFS recruiting
NCT04090528 2 Pembrolizumab + pTVG-HP (DNA vaccine) mCRPC PFS Active,
+ pTVG-AR HP (DNA vaccine) recruiting
Pembrolizumab + pTVG-HP
NCT04382898 1/2 Cemiplimab + BNT112 mCRPC after progression on 2-3 DLT, ORR, Safety Active,
BNT112 (RNA vaccine) therapies including NHA and/or recruiting
chemotherapy
ICIs + tyrosine kinase inhibitors
NCT04446117 3 Atezolizumab + Cabozantinib + NHA mCRPC after progression on 1 PFS, OS Active,
(Abiraterone or Enzalutamide) NHA recruiting
NCT03170960 1/2 Atezolimab + Carbozantinib mCRPC after progression on ≤ 1 DLT, ORR Active, not
NHA recruiting
NCT04477512 1 Nivolumab + Cabozantinib + Abiraterone mHSPC DLT Active,
recruiting
NCT04159896 2 Nivolumab + ESK981 (Pan-VEGFR/TIE2 TKI) mCRPC after progression on 1 Safety, PSA response Unknown
NHA and 1 chemotherapy
Combination ICIs
NCT04717154 2 Ipilimumab + Nivolumab mCRPC with immunogenic DCR Active,
signature recruiting
NCT03570619 2 Ipilimumab + Nivolumab mCRPC with CDK12 aberration ORR, PSA response Active, not
recruiting