Page 105 - Read Online
P. 105
Remley et al. Cancer Drug Resist 2023;6:748-67 https://dx.doi.org/10.20517/cdr.2023.63 Page 760
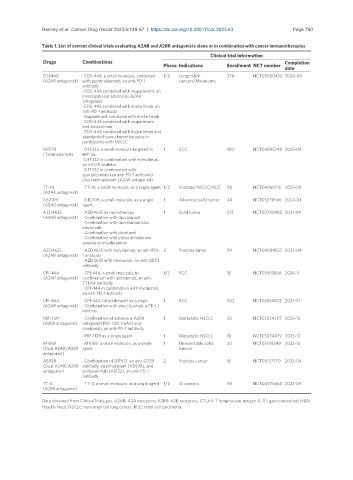
Table 1. List of current clinical trials evaluating A2AR and A2BR antagonists alone or in combination with cancer immunotherapies
Clinical trial information
Drugs Combinations Completion
Phase Indications Enrollment NCT number
date
EOS448 · EOS-448, a small molecule, combined 1/2 Lung/H&N 376 NCT05060432 2024-09
(A2AR antagonist) with pembrolizumab, an anti-PD-1 cancers/Melanoma
antibody
· EOS-448 combined with inupadenant, an
investigational adenosine A2AR
antagonist
· EOS-448 combined with dostarlimab, an
anti-PD-1 antibody
· Inupadenant combined with dostarlimab
· EOS-448 combined with inupadenant
and dostarlimab
· EOS-448 combined with dostarlimab and
standard-of-care chemotherapies in
participants with NSCLC
NIR178 · DFF332, a small molecule targeted to 1 RCC 180 NCT04895748 2025-04
(Taminadenant) HIF-2α
· DFF332 in combination with everolimus,
an mTOR inhibitor
· DFF332 in combination with
spartalizumab (an anti-PD-1 antibody)
plus taminadenant (A2AR antagonist)
TT-10 · TT-10, a small molecule, as a single agent 1/2 Prostate/NSCLC/RCC 90 NCT04969315 2025-08
(A2AR antagonist)
ILB2109 · ILB2109, a small molecule, as a single 1 Advanced solid tumor 48 NCT05278546 2024-01
(A2AR antagonist) agent
AZD4635 · AZD4635 as monotherapy 1 Solid tumor 313 NCT02740985 2021-04
(A2AR antagonist) · Combination with durvalumab
· Combination with durvalumab plus
oleclumab
· Combination with docetaxel
· Combination with either abiraterone
acetate or enzalutamide
AZD4635 · AZD4635 with durvalumab, an anti-PDL- 2 Prostate tumor 59 NCT04089553 2023-04
(A2AR antagonist) 1 antibody
· AZD4635 with oleclumab, an anti-CD73
antibody
CPI-444 · CPI-444, a small molecule, in 1/2 RCC 15 NCT05501054 2026-11
(A2AR antagonist) combination with ipilimumab, an anti-
CTLA4 antibody
· CPI-444 in combination with nivolumab,
an anti-PD-1 antibody
CPI-444 · CPI-444 (ciforadenant) as a single 1 RCC 502 NCT02655822 2021-07
(A2AR antagonist) · Combination with atezolizumab, a PD-L1
inhibitor
PBF-1129 · Combination of adenosine A2BR 1 Metastatic NSCLC 30 NCT05234307 2025-12
(A2BR antagonist) antagonist PBF-1129 (mAb) and
nivolumab, an anti-PD-1 antibody
· PBF-1129 as a single agent 1 Metastatic NSCLC 18 NCT03274479 2023-12
M1069 · M1069, a small molecule, as a single 1 Unresectable solid 30 NCT05198349 2023-12
(Dual A2AR/A2BR agent tumors
antagonist)
AB928 · Combination of SRF617, an anti-CD39 2 Prostate cancer 15 NCT05177770 2023-04
(Dual A2AR/A2BR antibody, etrumadenant (AB928), and
antagonist) zimberelimab (AB122), an anti-PD-1
antibody
TT-4 · TT-4, a small molecule, as a single agent 1/2 GI cancers 69 NCT04976660 2023-09
(A2BR antagonist)
Data obtained from ClinicalTrials.gov. A2AR: A2A receptors; A2BR: A2B receptors; CTLA4: T lymphocyte antigen 4; GI: gastrointestinal; H&N:
Head & Neck; NSCLC: non-small cell lung cancer; RCC: renal cell carcinoma.