Page 85 - Read Online
P. 85
Page 20 of 27 Liu et al. Microstructures 2023;3:2023020 https://dx.doi.org/10.20517/microstructures.2023.02
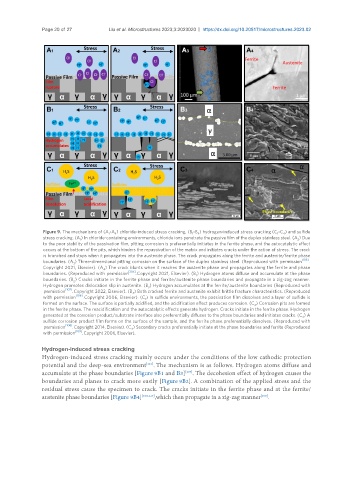
Figure 9. The mechanisms of (A -A ) chloride-induced stress cracking, (B -B ) hydrogen-induced stress cracking (C -C ) and sulfide
1
4
1
4
4
1
stress cracking. (A ) In chloride-containing environments, chloride ions penetrate the passive film of the duplex stainless steel. (A ) Due
1
2
to the poor stability of the passivation film, pitting corrosion is preferentially initiates in the ferrite phase, and the autocatalytic effect
occurs at the bottom of the pits, which hinders the repassivation of the matrix and initiates cracks under the action of stress. The crack
is branched and stops when it propagates into the austenite phase. The crack propagates along the ferrite and austenite/ferrite phase
boundaries. (A ) Three-dimensional pitting corrosion on the surface of the duplex stainless steel. (Reproduced with permission [105] .
3
Copyright 2021, Elsevier). (A ) The crack blunts when it reaches the austenite phase and propagates along the ferrite and phase
4
boundaries. (Reproduced with permission [105] . Copyright 2021, Elsevier). (B ) Hydrogen atoms diffuse and accumulate at the phase
1
boundaries. (B ) Cracks initiate in the ferrite phase and ferrite/austenite phase boundaries and propagate in a zig-zag manner.
2
Hydrogen promotes dislocation slip in austenite. (B ) Hydrogen accumulates at the ferrite/austenite boundaries (Reproduced with
3
permission [107] . Copyright 2022, Elsevier). (B ) Both cracked ferrite and austenite exhibit brittle fracture characteristics. (Reproduced
4
with permission [108] . Copyright 2006, Elsevier). (C ) In sulfide environments, the passivation film dissolves and a layer of sulfide is
1
formed on the surface. The surface is partially acidified, and the acidification effect produces corrosion. (C ) Corrosion pits are formed
2
in the ferrite phase. The reacidification and the autocatalytic effects generate hydrogen. Cracks initiate in the ferrite phase. Hydrogen
generated at the corrosion product/substrate interface also preferentially diffuses to the phase boundaries and initiates cracks. (C ) A
3
sulfide corrosion product film forms on the surface of the sample, and the ferrite phase preferentially dissolves. (Reproduced with
permission [106] . Copyright 2014, Elsevier). (C ) Secondary cracks preferentially initiate at the phase boundaries and ferrite (Reproduced
4
with permission [109] . Copyright 2006, Elsevier).
Hydrogen-induced stress cracking
Hydrogen-induced stress cracking mainly occurs under the conditions of the low cathodic protection
potential and the deep-sea environment . The mechanism is as follows. Hydrogen atoms diffuse and
[116]
accumulate at the phase boundaries [Figure 9B1 and B3] . The decohesion effect of hydrogen causes the
[107]
boundaries and planes to crack more easily [Figure 9B2]. A combination of the applied stress and the
residual stress cause the specimen to crack. The cracks initiate in the ferrite phase and at the ferrite/
austenite phase boundaries [Figure 9B4] [108,117] ,which then propagate in a zig-zag manner .
[108]