Page 83 - Read Online
P. 83
Page 18 of 27 Liu et al. Microstructures 2023;3:2023020 https://dx.doi.org/10.20517/microstructures.2023.02
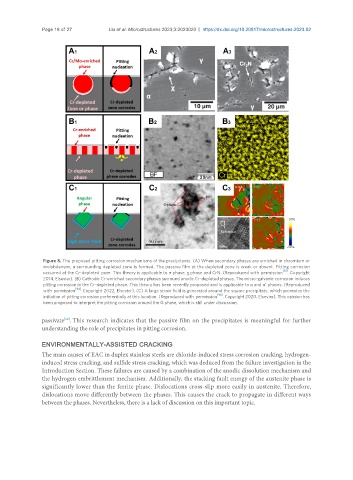
Figure 8. The proposed pitting corrosion mechanisms of the precipitants. (A) When secondary phases are enriched in chromium or
molybdenum, a surrounding depleted zone is formed. The passive film at the depleted zone is weak or absent. Pitting corrosion
[87]
occurred at the Cr-depleted zone. This theory is applicable to σ phase, χ phase and CrN. (Reproduced with permission . Copyright
2014, Elsevier). (B) Cathodic Cr-enriched secondary phases surround anodic Cr-depleted phases. The micro-galvanic corrosion induces
pitting corrosion in the Cr-depleted phase. This theory has been recently proposed and is applicable to α and α’ phases. (Reproduced
with permission [94] . Copyright 2022, Elsevier). (C) A large strain field is generated around the square precipitate, which promotes the
initiation of pitting corrosion preferentially at this location. (Reproduced with permission [96] . Copyright 2020, Elsevier). This opinion has
been proposed to interpret the pitting corrosion around the G phase, which is still under discussion.
passivate . This research indicates that the passive film on the precipitates is meaningful for further
[98]
understanding the role of precipitates in pitting corrosion.
ENVIRONMENTALLY-ASSISTED CRACKING
The main causes of EAC in duplex stainless steels are chloride-induced stress corrosion cracking, hydrogen-
induced stress cracking, and sulfide stress cracking, which was deduced from the failure investigation in the
Introduction Section. These failures are caused by a combination of the anodic dissolution mechanism and
the hydrogen embrittlement mechanism. Additionally, the stacking fault energy of the austenite phase is
significantly lower than the ferrite phase. Dislocations cross-slip more easily in austenite. Therefore,
dislocations move differently between the phases. This causes the crack to propagate in different ways
between the phases. Nevertheless, there is a lack of discussion on this important topic.