Page 79 - Read Online
P. 79
Page 14 of 27 Liu et al. Microstructures 2023;3:2023020 https://dx.doi.org/10.20517/microstructures.2023.02
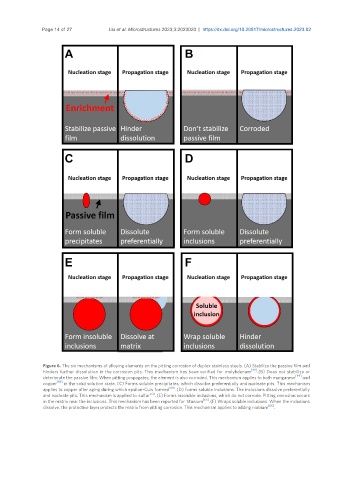
Figure 6. The six mechanisms of alloying elements on the pitting corrosion of duplex stainless steels. (A) Stabilize the passive film and
hinders further dissolution in the corrosion pits. This mechanism has been verified for molybdenum [49] . (B) Does not stabilize or
deteriorate the passive film. When pitting propagates, the element is also corroded. This mechanism applies to both manganese [49] and
copper [59] in the solid solution state. (C) Forms soluble precipitates, which dissolve preferentially and nucleate pits. This mechanism
applies to copper after aging during which epsilon-Cuis formed [60] . (D) Forms soluble inclusions. The inclusions dissolve preferentially
and nucleate pits. This mechanism is applied to sulfur [72] . (E) Forms insoluble inclusions, which do not corrode. Pitting corrosion occurs
in the matrix near the inclusions. This mechanism has been reported for titanium [65] . (F) Wraps soluble inclusions. When the inclusions
dissolve, the protective layer protects the matrix from pitting corrosion. This mechanism applies to adding niobium [66] .