Page 70 - Read Online
P. 70
Liu et al. Microstructures 2023;3:2023020 https://dx.doi.org/10.20517/microstructures.2023.02 Page 5 of 27
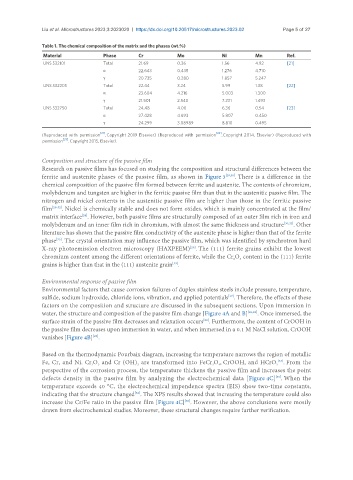
Table 1. The chemical composition of the matrix and the phases (wt.%)
Material Phase Cr Mo Ni Mn Ref.
UNS S32101 Total 21.69 0.36 1.56 4.92 [21]
α 22.643 0.435 1.276 4.710
γ 20.735 0.280 1.857 5.247
UNS S32205 Total 22.44 3.24 5.99 1.38 [22]
α 23.604 4.216 5.003 1.300
γ 21.501 2.540 7.231 1.493
UNS S32750 Total 24.48 4.00 6.36 0.54 [23]
α 27.428 4.693 5.807 0.450
γ 24.299 3.08989 8.810 0.495
(Reproduced with permission [21] . Copyright 2019 Elsevier) (Reproduced with permission [22] . Copyright 2014, Elsevier) (Reproduced with
permission [23] . Copyright 2015, Elsevier).
Composition and structure of the passive film
Research on passive films has focused on studying the composition and structural differences between the
ferrite and austenite phases of the passive film, as shown in Figure 3 [29,30] . There is a difference in the
chemical composition of the passive film formed between ferrite and austenite. The contents of chromium,
molybdenum and tungsten are higher in the ferritic passive film than that in the austenitic passive film. The
nitrogen and nickel contents in the austenitic passive film are higher than those in the ferritic passive
film [29-32] . Nickel is chemically stable and does not form oxides, which is mainly concentrated at the film/
matrix interface . However, both passive films are structurally composed of an outer film rich in iron and
[24]
molybdenum and an inner film rich in chromium, with almost the same thickness and structure [30,32] . Other
literature has shown that the passive film conductivity of the austenite phase is higher than that of the ferrite
phase . The crystal orientation may influence the passive film, which was identified by synchrotron hard
[33]
X-ray photoemission electron microscopy (HAXPEEM) . The (111) ferrite grains exhibit the lowest
[29]
chromium content among the different orientations of ferrite, while the Cr O content in the (111) ferrite
2
3
grains is higher than that in the (111) austenite grain .
[29]
Environmental response of passive film
Environmental factors that cause corrosion failures of duplex stainless steels include pressure, temperature,
sulfide, sodium hydroxide, chloride ions, vibration, and applied potentials . Therefore, the effects of these
[17]
factors on the composition and structure are discussed in the subsequent sections. Upon immersion in
water, the structure and composition of the passive film change [Figure 4A and B] [26,28] . Once immersed, the
surface strain of the passive film decreases and relaxation occurs . Furthermore, the content of CrOOH in
[28]
the passive film decreases upon immersion in water, and when immersed in a 0.1 M NaCl solution, CrOOH
vanishes [Figure 4B] .
[28]
Based on the thermodynamic Pourbaix diagram, increasing the temperature narrows the region of metallic
Fe, Cr, and Ni. Cr O and Cr (OH) are transformed into FeCr O , CrOOH, and HCrO . From the
[34]
4
2
3
2
2
3
perspective of the corrosion process, the temperature thickens the passive film and increases the point
defects density in the passive film by analyzing the electrochemical data [Figure 4C] . When the
[35]
temperature exceeds 40 °C, the electrochemical impendence spectra (EIS) show two-time constants,
indicating that the structure changed . The XPS results showed that increasing the temperature could also
[36]
increase the Cr/Fe ratio in the passive film [Figure 4C] . However, the above conclusions were mostly
[36]
drawn from electrochemical studies. Moreover, these structural changes require further verification.