Page 135 - Read Online
P. 135
Page 4 of 31 Chen et al. Microstructures 2023;3:2023025 https://dx.doi.org/10.20517/microstructures.2023.12
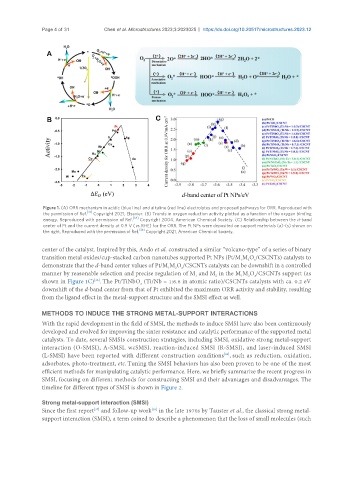
Figure 1. (A) ORR mechanism in acidic (blue line) and alkaline (red line) electrolytes and proposed pathways for ORR. Reproduced with
[31]
the permission of Ref. Copyright 2021, Elsevier. (B) Trends in oxygen reduction activity plotted as a function of the oxygen binding
[34]
energy. Reproduced with permission of Ref. Copyright 2004, American Chemical Society. (C) Relationship between the d-band
center of Pt and the current density at 0.9 V (vs. RHE) for the ORR. The Pt NPs were deposited on support materials (a)-(s) shown on
[36]
the right. Reproduced with the permission of Ref. Copyright 2021, American Chemical Society.
center of the catalyst. Inspired by this, Ando et al. constructed a similar “volcano-type” of a series of binary
transition metal oxides/cup-stacked carbon nanotubes supported Pt NPs (Pt/M M O /CSCNTs) catalysts to
2
x
1
demonstrate that the d-band center values of Pt/M M O /CSCNTs catalysts can be downshift in a controlled
1
x
2
manner by reasonable selection and precise regulation of M and M in the M M O /CSCNTs support (as
2
1
2
1
x
[36]
shown in Figure 1C) . The Pt/TiNbO (Ti/Nb = 1:6.6 in atomic ratio)/CSCNTs catalysts with ca. 0.2 eV
x
downshift of the d-band center from that of Pt exhibited the maximum ORR activity and stability, resulting
from the ligand effect in the metal-support structure and the SMSI effect as well.
METHODS TO INDUCE THE STRONG METAL-SUPPORT INTERACTIONS
With the rapid development in the field of SMSI, the methods to induce SMSI have also been continuously
developed and evolved for improving the sinter resistance and catalytic performance of the supported metal
catalysts. To date, several SMSIs construction strategies, including SMSI, oxidative strong metal-support
interaction (O-SMSI), A-SMSI, wcSMSI, reaction-induced SMSI (R-SMSI), and laser-induced SMSI
(L-SMSI) have been reported with different construction conditions , such as reduction, oxidation,
[26]
adsorbates, photo-treatment, etc. Tuning the SMSI behaviors has also been proven to be one of the most
efficient methods for manipulating catalytic performance. Here, we briefly summarize the recent progress in
SMSI, focusing on different methods for constructing SMSI and their advantages and disadvantages. The
timeline for different types of SMSI is shown in Figure 2.
Strong metal-support interaction (SMSI)
Since the first report and follow-up work in the late 1970s by Tauster et al., the classical strong metal-
[37]
[38]
support interaction (SMSI), a term coined to describe a phenomenon that the loss of small molecules (such