Page 98 - Read Online
P. 98
Page 18 of 26 Yang et al. Soft Sci 2024;4:9 https://dx.doi.org/10.20517/ss.2023.43
3+
3+
3+
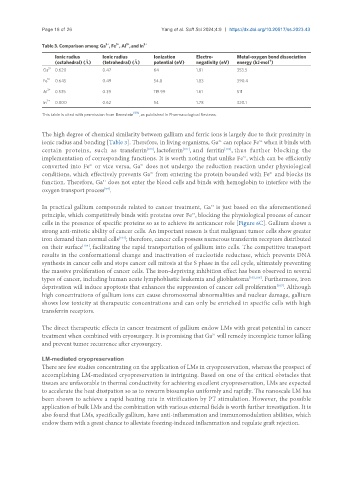
Table 3. Comparison among Ga , Fe , Al , and In 3+
Ionic radius Ionic radius Ionization Electro- Metal-oxygen bond dissociation
-1
(octahedral) (Å) (tetrahedral) (Å) potential (eV) negativity (eV) energy (kJ·mol )
Ga 3+ 0.620 0.47 64 1.81 353.5
Fe 3+ 0.645 0.49 54.8 1.83 390.4
Al 3+ 0.535 0.39 119.99 1.61 511
3+
In 0.800 0.62 54 1.78 320.1
[99]
This table is cited with permission from Bernstein , as published in Pharmacological Reviews.
The high degree of chemical similarity between gallium and ferric ions is largely due to their proximity in
3+
ionic radius and bonding [Table 3]. Therefore, in living organisms, Ga can replace Fe when it binds with
3+
certain proteins, such as transferrin , lactoferrin , and ferritin , thus further blocking the
[102]
[101]
[100]
implementation of corresponding functions. It is worth noting that unlike Fe , which can be efficiently
3+
converted into Fe or vice versa, Ga does not undergo the reduction reaction under physiological
3+
2+
3+
conditions, which effectively prevents Ga from entering the protein bounded with Fe and blocks its
2+
function. Therefore, Ga does not enter the blood cells and binds with hemoglobin to interfere with the
3+
oxygen transport process .
[99]
3+
In practical gallium compounds related to cancer treatment, Ga is just based on the aforementioned
3+
principle, which competitively binds with proteins over Fe , blocking the physiological process of cancer
cells in the presence of specific proteins so as to achieve its anticancer role [Figure 6C]. Gallium shows a
strong anti-mitotic ability of cancer cells. An important reason is that malignant tumor cells show greater
[103]
iron demand than normal cells ; therefore, cancer cells possess numerous transferrin receptors distributed
on their surface , facilitating the rapid transportation of gallium into cells. The competitive transport
[104]
results in the conformational change and inactivation of nucleotide reductase, which prevents DNA
synthesis in cancer cells and stops cancer cell mitosis at the S phase in the cell cycle, ultimately preventing
the massive proliferation of cancer cells. The iron-depriving inhibition effect has been observed in several
types of cancer, including human acute lymphoblastic leukemia and glioblastoma [105,106] . Furthermore, iron
deprivation will induce apoptosis that enhances the suppression of cancer cell proliferation . Although
[107]
high concentrations of gallium ions can cause chromosomal abnormalities and nuclear damage, gallium
shows low toxicity at therapeutic concentrations and can only be enriched in specific cells with high
transferrin receptors.
The direct therapeutic effects in cancer treatment of gallium endow LMs with great potential in cancer
treatment when combined with cryosurgery. It is promising that Ga will remedy incomplete tumor killing
3+
and prevent tumor recurrence after cryosurgery.
LM-mediated cryopreservation
There are few studies concentrating on the application of LMs in cryopreservation, whereas the prospect of
accomplishing LM-mediated cryopreservation is intriguing. Based on one of the critical obstacles that
tissues are unfavorable in thermal conductivity for achieving excellent cryopreservation, LMs are expected
to accelerate the heat dissipation so as to rewarm biosamples uniformly and rapidly. The nanoscale LM has
been shown to achieve a rapid heating rate in vitrification by PT stimulation. However, the possible
application of bulk LMs and the combination with various external fields is worth further investigation. It is
also found that LMs, specifically gallium, have anti-inflammation and immunomodulation abilities, which
endow them with a great chance to alleviate freezing-induced inflammation and regulate graft rejection.