Page 111 - Read Online
P. 111
Zhang et al. Soft Sci 2024;4:23 https://dx.doi.org/10.20517/ss.2023.58 Page 5 of 21
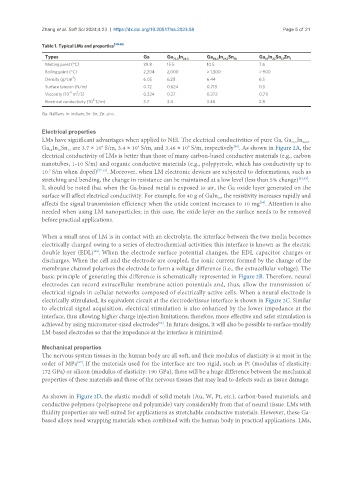
Table 1. Typical LMs and properties [48-55]
Types Ga Ga 75.5 In 24.5 Ga 68.5 In 21.5 Sn 10 Ga In Sn Zn 1
61
25
13
Melting point (°C) 29.8 15.5 10.5 7.6
Boiling point (°C) 2,204 2,000 > 1,300 > 900
3
Density (g/cm ) 6.05 6.28 6.44 6.5
Surface tension (N/m) 0.72 0.624 0.718 0.5
-6 2
Viscosity (10 m /S) 0.324 0.27 0.372 0.711
6
Electrical conductivity (10 S/m) 3.7 3.4 3.46 2.8
Ga: Gallium; In: indium; Sn: tin; Zn: zinc.
Electrical properties
LMs have significant advantages when applied to NEI. The electrical conductivities of pure Ga, Ga In ,
24.5
75.5
Ga In Sn are 3.7 × 10 S/m, 3.4 × 10 S/m, and 3.46 × 10 S/m, respectively . As shown in Figure 2A, the
[56]
6
6
6
12
20
68
electrical conductivity of LMs is better than those of many carbon-based conductive materials (e.g., carbon
nanotubes, 1-10 S/m) and organic conductive materials (e.g., polypyrrole, which has conductivity up to
10 S/m when doped) [57-61] . Moreover, when LM electronic devices are subjected to deformations, such as
-5
stretching and bending, the change in resistance can be maintained at a low level (less than 5% change) [62,63] .
It should be noted that when the Ga-based metal is exposed to air, the Ga oxide layer generated on the
surface will affect electrical conductivity. For example, for 40 g of GaIn , the resistivity increases rapidly and
10
affects the signal transmission efficiency when the oxide content increases to 10 mg . Attention is also
[64]
needed when using LM nanoparticles; in this case, the oxide layer on the surface needs to be removed
before practical applications.
When a small area of LM is in contact with an electrolyte, the interface between the two media becomes
electrically charged owing to a series of electrochemical activities; this interface is known as the electric
double layer (EDL) . When the electrode surface potential changes, the EDL capacitor charges or
[65]
discharges. When the cell and the electrode are coupled, the ionic current formed by the change of the
membrane channel polarizes the electrode to form a voltage difference (i.e., the extracellular voltage). The
basic principle of generating this difference is schematically represented in Figure 2B. Therefore, neural
electrodes can record extracellular membrane action potentials and, thus, allow the transmission of
electrical signals in cellular networks composed of electrically active cells. When a neural electrode is
electrically stimulated, its equivalent circuit at the electrode/tissue interface is shown in Figure 2C. Similar
to electrical signal acquisition, electrical stimulation is also enhanced by the lower impedance at the
interface, thus allowing higher charge injection limitations; therefore, more effective and safer stimulation is
achieved by using micrometer-sized electrodes . In future designs, it will also be possible to surface-modify
[66]
LM-based electrodes so that the impedance at the interface is minimized.
Mechanical properties
The nervous system tissues in the human body are all soft, and their modulus of elasticity is at most in the
order of MPa . If the materials used for the interface are too rigid, such as Pt (modulus of elasticity:
[67]
172 GPa) or silicon (modulus of elasticity: 190 GPa), there will be a huge difference between the mechanical
properties of these materials and those of the nervous tissues that may lead to defects such as tissue damage.
As shown in Figure 2D, the elastic moduli of solid metals (Au, W, Pt, etc.), carbon-based materials, and
conductive polymers (polyisoprene and polyamide) vary considerably from that of neural tissue. LMs with
fluidity properties are well suited for applications as stretchable conductive materials. However, these Ga-
based alloys need wrapping materials when combined with the human body in practical applications. LMs,