Page 96 - Read Online
P. 96
Page 8 of 34 West et al. Rare Dis Orphan Drugs J 2024;3:22 https://dx.doi.org/10.20517/rdodj.2023.61
Table 1. Pitfalls in the diagnosis of Fabry nephropathy
Reference
Low α-Gal activity in patients without Fabry disease on hemodialysis Nakao et al., 2003 [43]
Low α-Gal activity reported in 9% of patients with FSGS but without Fabry disease Hasbal et al., 2020 [44]
[45]
Abnormal elevation sphingolipids reported in patients with FSGS and diabetic nephropathy Mersher et al., 2014
Increased urine glycosphingolipids in patients with chronic glomerulonephritis Townsend et al., 1978 [46]
[47]
Elevated urine Gb3 in men with nephrotic syndrome without Fabry disease West et al., 2012
Increased plasma lyso-Gb3 in other lysosomal diseases Ferraz et al., 2016 [48]
α-Gal: Alpha-galactosidase A; FSGS: focal segmental glomerulosclerosis; Gb3: globotriaosylceramide; lyso-Gb3: globotriaosylsphingosine.
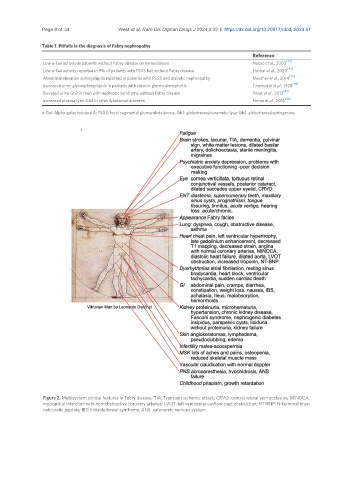
Figure 2. Multisystem clinical features in Fabry disease. TIA: Transient ischemic attack; CRVO: central retinal vein occlusion; MINOCA:
myocardial infarction with non-obstructive coronary arteries; LVOT: left ventricular outflow tract obstruction; NT-BNP: N-terminal brain
natriuretic peptide; IBS: irritable bowel syndrome; ANS: autonomic nervous system.