Page 717 - Read Online
P. 717
Meltzer et al. Plast Aesthet Res 2020;7:61 I http://dx.doi.org/10.20517/2347-9264.2020.122 Page 5 of 12
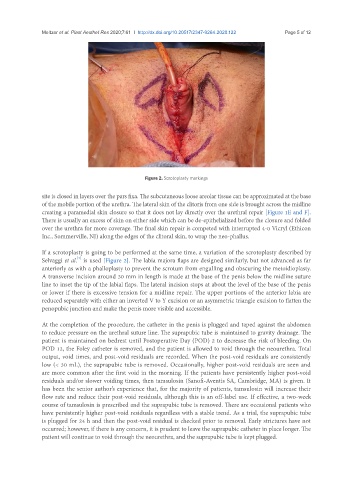
Figure 2. Scrotoplasty markings
site is closed in layers over the pars fixa. The subcutaneous loose areolar tissue can be approximated at the base
of the mobile portion of the urethra. The lateral skin of the clitoris from one side is brought across the midline
creating a paramedial skin closure so that it does not lay directly over the urethral repair [Figure 1E and F].
There is usually an excess of skin on either side which can be de-epithelialized before the closure and folded
over the urethra for more coverage. The final skin repair is competed with interrupted 4-0 Vicryl (Ethicon
Inc., Sommerville, NJ) along the edges of the clitoral skin, to wrap the neo-phallus.
If a scrotoplasty is going to be performed at the same time, a variation of the scrotoplasty described by
[7]
Selvaggi et al. is used [Figure 2]. The labia majora flaps are designed similarly, but not advanced as far
anteriorly as with a phalloplasty to prevent the scrotum from engulfing and obscuring the metoidioplasty.
A transverse incision around 30 mm in length is made at the base of the penis below the midline suture
line to inset the tip of the labial flaps. The lateral incision stops at about the level of the base of the penis
or lower if there is excessive tension for a midline repair. The upper portions of the anterior labia are
reduced separately with either an inverted V to Y excision or an asymmetric triangle excision to flatten the
penopubic junction and make the penis more visible and accessible.
At the completion of the procedure, the catheter in the penis is plugged and taped against the abdomen
to reduce pressure on the urethral suture line. The suprapubic tube is maintained to gravity drainage. The
patient is maintained on bedrest until Postoperative Day (POD) 2 to decrease the risk of bleeding. On
POD 12, the Foley catheter is removed, and the patient is allowed to void through the neourethra. Total
output, void times, and post-void residuals are recorded. When the post-void residuals are consistently
low (< 30 mL), the suprapubic tube is removed. Occasionally, higher post-void residuals are seen and
are more common after the first void in the morning. If the patients have persistently higher post-void
residuals and/or slower voiding times, then tamsulosin (Sanofi-Aventis SA, Cambridge, MA) is given. It
has been the senior author’s experience that, for the majority of patients, tamsulosin will increase their
flow rate and reduce their post-void residuals, although this is an off-label use. If effective, a two-week
course of tamsulosin is prescribed and the suprapubic tube is removed. There are occasional patients who
have persistently higher post-void residuals regardless with a stable trend. As a trial, the suprapubic tube
is plugged for 24 h and then the post-void residual is checked prior to removal. Early strictures have not
occurred; however, if there is any concern, it is prudent to leave the suprapubic catheter in place longer. The
patient will continue to void through the neourethra, and the suprapubic tube is kept plugged.