Page 616 - Read Online
P. 616
Page 8 of 13 Ziai et al. Plast Aesthet Res 2020;7:53 I http://dx.doi.org/10.20517/2347-9264.2020.151
A B
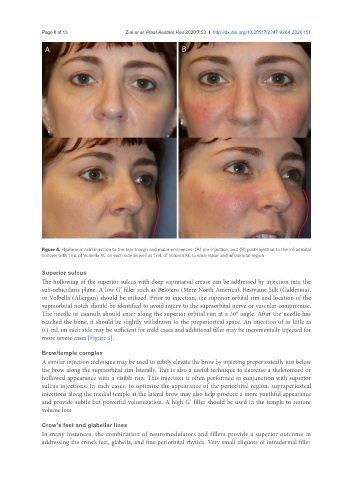
Figure 4. Hyaluronic acid injection to the tear trough and malar eminences: (A) pre-injection; and (B) post-injection to the infraorbital
hollows with 1 mL of Volbella XC on each side as well as 1 mL of Voluma XC to each malar and infraorbital region
Superior sulcus
The hollowing of the superior sulcus with deep supratarsal crease can be addressed by injection into the
sub-orbicularis plane. A low G’ filler such as Belotero (Merz North America), Restylane Silk (Galderma),
or Volbella (Allergan) should be utilized. Prior to injection, the superior orbital rim and location of the
supraorbital notch should be identified to avoid injury to the supraorbital nerve or vascular compromise.
The needle or cannula should enter along the superior orbital rim at a 30° angle. After the needle has
reached the bone, it should be slightly withdrawn to the preperiosteal space. An injection of as little as
0.1 mL on each side may be sufficient for mild cases and additional filler may be incrementally injected for
more severe cases [Figure 5].
Brow/temple complex
A similar injection technique may be used to subtly elevate the brow by injecting preperiosteally just below
the brow along the supraorbital rim laterally. This is also a useful technique to decrease a skeletonized or
hollowed appearance with a visible rim. This injection is often performed in conjunction with superior
sulcus injections. In such cases, to optimize the appearance of the periorbital region, supraperiosteal
injections along the medial temple at the lateral brow may also help produce a more youthful appearance
and provide subtle but powerful volumization. A high G’ filler should be used in the temple to restore
volume loss.
Crow’s feet and glabellar lines
In many instances, the combination of neuromodulators and fillers provide a superior outcome in
addressing the crow’s feet, glabella, and fine periorbital rhytids. Very small aliquots of intradermal filler