Page 61 - Read Online
P. 61
Alimi et al. Plast Aesthet Res 2020;7:5 I http://dx.doi.org/10.20517/2347-9264.2019.39 Page 7 of 9
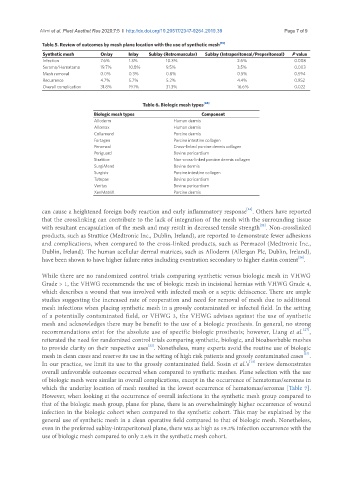
Table 5. Review of outcomes by mesh plane location with the use of synthetic mesh [15]
Synthetic mesh Onlay Inlay Sublay (Retromuscular) Sublay (Intraperitoneal/Preperitoneal) P value
Infection 7.6% 1.3% 10.3% 2.6% 0.008
Seroma/Hematoma 19.7% 10.8% 9.5% 3.5% 0.003
Mesh removal 0.0% 0.3% 0.8% 0.5% 0.594
Recurrence 4.7% 5.7% 5.2% 4.4% 0.952
Overall complication 31.8% 19.1% 31.3% 16.6% 0.022
Table 6. Biologic mesh types [24]
Biologic mesh types Component
Alloderm Human dermis
Allomax Human dermis
Collamend Porcine dermis
Fortagen Porcine intestine collagen
Peramcol Cross-linked porcine dermis collagen
Periguard Bovine pericardium
Strattice Non-cross-linked porcine dermis collagen
SurgiMend Bovine dermis
Surgisis Porcine intestine collagen
Tutopas Bovine pericardium
Veritas Bovine pericardium
XenMatriX Porcine dermis
can cause a heightened foreign body reaction and early inflammatory response . Others have reported
[24]
that the crosslinking can contribute to the lack of integration of the mesh with the surrounding tissue
[25]
with resultant encapsulation of the mesh and may result in decreased tensile strength . Non-crosslinked
products, such as Strattice (Medtronic Inc., Dublin, Ireland), are reported to demonstrate fewer adhesions
and complications, when compared to the cross-linked products, such as Permacol (Medtronic Inc.,
Dublin, Ireland). The human acellular dermal matrices, such as Alloderm (Allergan Plc, Dublin, Ireland),
have been shown to have higher failure rates including eventration secondary to higher elastin content .
[26]
While there are no randomized control trials comparing synthetic versus biologic mesh in VHWG
Grade > 1, the VHWG recommends the use of biologic mesh in incisional hernias with VHWG Grade 4,
which describes a wound that was involved with infected mesh or a septic dehiscence. There are ample
studies suggesting the increased rate of reoperation and need for removal of mesh due to additional
mesh infections when placing synthetic mesh in a grossly contaminated or infected field. In the setting
of a potentially contaminated field, or VHWG 3, the VHWG advises against the use of synthetic
mesh and acknowledges there may be benefit to the use of a biologic prosthesis. In general, no strong
[27]
recommendations exist for the absolute use of specific biologic prosthesis; however, Liang et al. ,
reiterated the need for randomized control trials comparing synthetic, biologic, and bioabsorbable meshes
[22]
to provide clarity on their respective uses . Nonetheless, many experts avoid the routine use of biologic
[27]
mesh in clean cases and reserve its use in the setting of high risk patients and grossly contaminated cases .
[15]
In our practice, we limit its use to the grossly contaminated field. Sosin et al.’s review demonstrates
overall unfavorable outcomes occurred when compared to synthetic meshes. Plane selection with the use
of biologic mesh were similar in overall complications, except in the occurrence of hematomas/seromas in
which the underlay location of mesh resulted in the lowest occurrence of hematomas/seromas [Table 7].
However, when looking at the occurrence of overall infections in the synthetic mesh group compared to
that of the biologic mesh group, plane for plane, there is an overwhelmingly higher occurrence of wound
infection in the biologic cohort when compared to the synthetic cohort. This may be explained by the
general use of synthetic mesh in a clean operative field compared to that of biologic mesh. Nonetheless,
even in the preferred sublay-intraperitoneal plane, there was as high as 19.2% infection occurrence with the
use of biologic mesh compared to only 2.6% in the synthetic mesh cohort.