Page 59 - Read Online
P. 59
Alimi et al. Plast Aesthet Res 2020;7:5 I http://dx.doi.org/10.20517/2347-9264.2019.39 Page 5 of 9
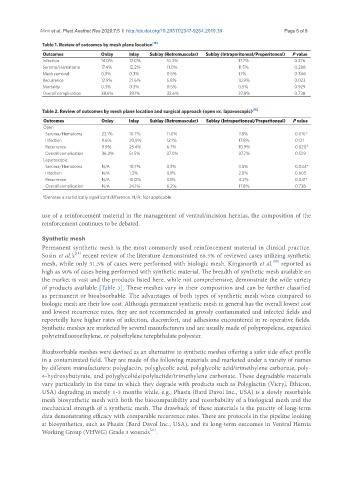
Table 1. Review of outcomes by mesh plane location [15]
Outcomes Onlay Inlay Sublay (Retromuscular) Sublay (Intraperitoneal/Preperitoneal) P value
Infection 14.0% 12.0% 10.2% 17.7% 0.276
Seroma/Hematoma 17.4% 12.2% 11.0% 11.5% 0.288
Mesh removal 0.3% 0.3% 0.5% 1.1% 0.346
Recurrence 12.9% 21.6% 5.8% 10.9% 0.023
Mortality 0.3% 0.3% 0.5% 0.5% 0.929
Overall complication 38.6% 39.1% 32.6% 37.8% 0.738
Table 2. Review of outcomes by mesh plane location and surgical approach (open vs. laparoscopic) [15]
Outcomes Onlay Inlay Sublay (Retromuscular) Sublay (Intraperitoneal/Preperitoneal) P value
Open
Seroma/Hematoma 22.1% 10.7% 11.0% 7.8% 0.016*
Infection 9.6% 20.9% 12.1% 17.8% 0.121
Recurrence 9.9% 25.4% 6.7% 10.9% 0.020*
Overall complication 36.2% 51.5% 37.0% 37.7% 0.529
Laparoscopic
Seroma/Hematoma N/A 10.7% 3.3% 3.5% 0.044*
Infection N/A 1.3% 0.1% 2.8% 0.605
Recurrence N/A 10.0% 0.1% 4.2% 0.041*
Overall complication N/A 24.1% 6.2% 17.8% 0.738
*Denotes a statistically significant difference. N/A: Not applicable
use of a reinforcement material in the management of ventral/incision hernias, the composition of the
reinforcement continues to be debated.
Synthetic mesh
Permanent synthetic mesh is the most commonly used reinforcement material in clinical practice.
Sosin et al.’s recent review of the literature demonstrated 68.5% of reviewed cases utilizing synthetic
[15]
[20]
mesh, while only 31.5% of cases were performed with biologic mesh. Kingsnorth et al. reported as
high as 90% of cases being performed with synthetic material. The breadth of synthetic mesh available on
the market is vast and the products listed here, while not comprehensive, demonstrate the wide variety
of products available [Table 3]. These meshes vary in their composition and can be further classified
as permanent or bioabsorbable. The advantages of both types of synthetic mesh when compared to
biologic mesh are their low cost. Although permanent synthetic mesh in general has the overall lowest cost
and lowest recurrence rates, they are not recommended in grossly contaminated and infected fields and
reportedly have higher rates of infection, discomfort, and adhesions encountered in re-operative fields.
Synthetic meshes are marketed by several manufacturers and are usually made of polypropelene, expanded
polytetrafluoroethylene, or polyethylene terephthalate polyester.
Bioabsorbable meshes were devised as an alternative to synthetic meshes offering a safer side effect profile
in a contaminated field. They are made of the following materials and marketed under a variety of names
by different manufacturers: polyglactin, polyglycolic acid, polyglycolic acid/trimethylene carbonate, poly-
4-hydroxybutyrate, and polyglycolide/polylactide/trimethylene carbonate. These degradable materials
vary particularly in the time in which they degrade with products such as Polyglactin (Vicryl, Ethicon,
USA) degrading in merely 1-3 months while, e.g., Phasix (Bard Davol Inc., USA) is a slowly resorbable
mesh biosynthetic mesh with both the biocompatibility and resorbability of a biological mesh and the
mechanical strength of a synthetic mesh. The drawback of these materials is the paucity of long-term
data demonstrating efficacy with comparable recurrence rates. There are protocols in the pipeline looking
at biosynthetics, such as Phasix (Bard Davol Inc., USA), and its long-term outcomes in Ventral Hernia
Working Group (VHWG) Grade 3 wounds .
[21]