Page 265 - Read Online
P. 265
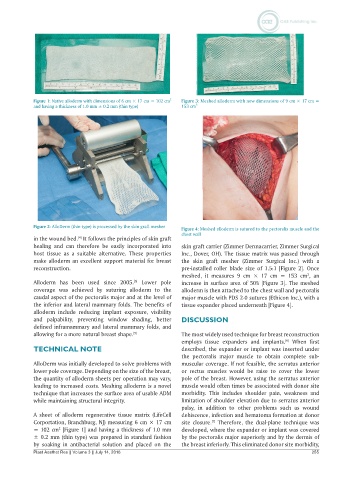
Figure 1: Native alloderm with dimensions of 6 cm × 17 cm = 102 cm 2 Figure 3: Meshed alloderm with new dimensions of 9 cm × 17 cm =
and having a thickness of 1.0 mm ± 0.2 mm (thin type) 153 cm 2
Figure 2: AlloDerm (thin-type) is processed by the skin graft mesher Figure 4: Meshed alloderm is sutured to the pectoralis muscle and the
chest wall
in the wound bed. It follows the principles of skin graft
[4]
healing and can therefore be easily incorporated into skin graft carrier (Zimmer Dermacarrier, Zimmer Surgical
host tissue as a suitable alternative. These properties Inc., Dover, OH). The tissue matrix was passed through
make alloderm an excellent support material for breast the skin graft mesher (Zimmer Surgical Inc.) with a
reconstruction. pre-installed roller blade size of 1.5:1 [Figure 2]. Once
meshed, it measures 9 cm × 17 cm = 153 cm , an
2
Alloderm has been used since 2005. Lower pole increase in surface area of 50% [Figure 3]. The meshed
[5]
coverage was achieved by suturing alloderm to the alloderm is then attached to the chest wall and pectoralis
caudal aspect of the pectoralis major and at the level of major muscle with PDS 2-0 sutures (Ethicon Inc.), with a
the inferior and lateral mammary folds. The benefits of tissue expander placed underneath [Figure 4].
alloderm include reducing implant exposure, visibility
and palpability, preventing window shading, better DISCUSSION
defined inframammary and lateral mammary folds, and
allowing for a more natural breast shape. [5] The most widely used technique for breast reconstruction
employs tissue expanders and implants. When first
[6]
TECHNICAL NOTE described, the expander or implant was inserted under
the pectoralis major muscle to obtain complete sub-
AlloDerm was initially developed to solve problems with muscular coverage. If not feasible, the serratus anterior
lower pole coverage. Depending on the size of the breast, or rectus muscles would be raise to cover the lower
the quantity of alloderm sheets per operation may vary, pole of the breast. However, using the serratus anterior
leading to increased costs. Meshing alloderm is a novel muscle would often times be associated with donor site
technique that increases the surface area of usable ADM morbidity. This includes shoulder pain, weakness and
while maintaining structural integrity. limitation of shoulder elevation due to serratus anterior
palsy, in addition to other problems such as wound
A sheet of alloderm regenerative tissue matrix (LifeCell dehiscence, infection and hematoma formation at donor
Corportation, Branchburg, NJ) measuring 6 cm × 17 cm site closure. Therefore, the dual-plane technique was
[7]
2
= 102 cm [Figure 1] and having a thickness of 1.0 mm developed, where the expander or implant was covered
± 0.2 mm (thin type) was prepared in standard fashion by the pectoralis major superiorly and by the dermis of
by soaking in antibacterial solution and placed on the the breast inferiorly. This eliminated donor site morbidity,
Plast Aesthet Res || Volume 3 || July 14, 2016 255