Page 13 - Read Online
P. 13
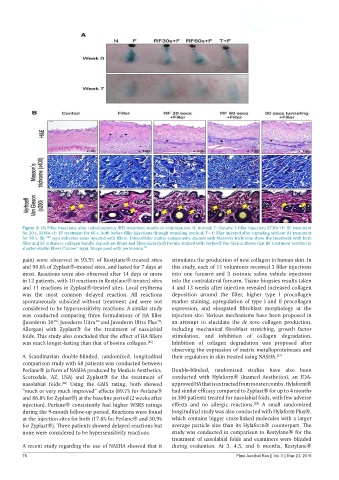
Figure 2: (A) Filler injections after radiofrequency (RF) treatment results in volumization. N: normal; F: Glytone 3 filler injection; RF30s+F: RF treatment
for 30 s, RF60s+F: RF treatment for 60 s, both before filler injections through tunneling method; T+F: filler injected after tunneling without RF treatment
for 30 s. (B) “*” sign indicates areas injected with fillers. Extracellular matrix components stained with Masson's trichrome show the treatment with both
filler and RF enhances collagen bundle deposition (blue) and fibroplasia (red) Dermis stained with Verhoeff-Van Gieson shows that RF treatment resulted in
shorter elastin fibers (“arrow” sign). Image used with permission. [3]
pain) were observed in 93.5% of Restylane®-treated sites stimulates the production of new collagen in human skin. In
and 90.6% of Zyplast®-treated sites, and lasted for 7 days at this study, each of 11 volunteers received 3 filler injections
most. Reactions were also observed after 14 days or more into one forearm and 3 isotonic saline vehicle injections
in 12 patients, with 10 reactions in Restylane®-treated sites into the contralateral forearm. Tissue biopsies results taken
and 11 reactions in Zyplast®-treated sites. Local erythema 4 and 13 weeks after injection revealed increased collagen
was the most common delayed reaction. All reactions deposition around the filler, higher type I procollagen
spontaneously subsided without treatment and were not marker staining, upregulation of type I and II procollagen
considered to be hypersensitivity reactions. A similar study expression, and elongated fibroblast morphology at the
was conducted comparing three formulations of HA filler injection site. Various mechanisms have been proposed in
(Juvederm 30™, Juvederm Ultra™ and Juvederm Ultra Plus™; an attempt to elucidate the de novo collagen production,
Allergan) with Zyplast® for the treatment of nasolabial including mechanical fibroblast stretching, growth factor
folds. This study also concluded that the effect of HA fillers stimulation, and inhibition of collagen degradation.
was much longer-lasting than that of bovine collagen. [16] Inhibition of collagen degradation was proposed after
observing the expression of matrix metalloproteinases and
A Scandinavian double-blinded, randomized, longitudinal their regulators in skin treated using NASHA. [27]
comparison study with 68 patients was conducted between
Perlane® (a form of NASHA produced by Medicis Aesthetics, Double-blinded, randomized studies have also been
Scottsdale, AZ, USA) and Zyplast® for the treatment of conducted with Hylaform® (Inamed Aesthetics), an FDA-
nasolabial folds. Using the GAIS rating, both showed approved HA that is extracted from rooster combs. Hylaform®
[26]
“much or very much improved” effects (89.7% for Perlane® had similar efficacy compared to Zyplast® for up to 4 months
and 86.8% for Zyplast®) at the baseline period (2 weeks after in 300 patients treated for nasolabial folds, with few adverse
injection). Perlane® consistently had higher WSRS ratings effects and no allergic reactions. A small randomized
[28]
during the 9-month follow-up period. Reactions were found longitudinal study was also conducted with Hylaform Plus®,
at the injection sites for both (17.6% for Perlane® and 30.9% which contains bigger cross-linked molecules with a larger
for Zyplast®). Three patients showed delayed reactions but average particle size than its Hylaform® counterpart. The
none were considered to be hypersensitivity reactions. study was conducted in comparison to Restylane® for the
treatment of nasolabial folds and examiners were blinded
A recent study regarding the use of NASHA showed that it during evaluation. At 3, 4.5, and 6 months, Restylane®
76 Plast Aesthet Res || Vol 3 || Mar 23, 2016