Page 11 - Read Online
P. 11
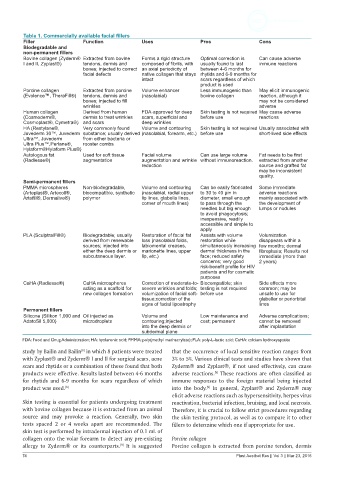
Table 1. Commercially available facial fillers
Filler Function Uses Pros Cons
Biodegradable and
non-permanent fillers
Bovine collagen (Zyderm® Extracted from bovine Forms a rigid structure Optimal correction is Can cause adverse
I and II, Zyplast®) tendons, dermis and composed of fibrils, with usually found to last immune reactions
bones; injected to correct an axial periodicity of between 4-6 months for
facial defects native collagen that stays rhytids and 6-9 months for
intact scars regardless of which
product is used
Porcine collagen Extracted from porcine Volume enhancer Less immunogenic than May elicit immunogenic
(Evolence™, TheraFill®) tendons, dermis and (nasolabial) bovine collagen reaction, although it
bones; injected to fill may not be considered
wrinkles adverse
Human collagen Derived from human FDA-approved for deep Skin testing is not required May cause adverse
(Cosmoderm®, dermis to treat wrinkles scars, superficial and before use reactions
Cosmoplast®, Cymetra®) and scars deep wrinkles
HA (Restylane®, Very commonly found Volume and contouring Skin testing is not required Usually associated with
Juvederm 30™, Juvederm substance; usually derived (nasolabial, forearm, etc.) before use short-lived side effects
Ultra™, Juvederm from either bacteria or
Ultra Plus™,Perlane®, rooster combs
Hylaform®Hylaform Plus®)
Autologous fat Used for soft tissue Facial volume Can use large volume Fat needs to be first
(Radiesse®) augmentation augmentation and wrinkle without immunoreaction. extracted from another
reduction source and grafted fat
may be inconsistent
quality.
Semi-permanent fillers
PMMA microspheres Non-biodegradable, Volume and contouring Can be easily fabricated Some immediate
(Arteplast®, Artecoll®, biocompatible, synthetic (nasolabial, radial upper to 30 to 40 μm in adverse reactions
Artefill®, Dermalive®) polymer lip lines, glabella lines, diameter, small enough mainly associated with
corner of mouth lines) to pass through the the development of
needles but big enough lumps or nodules
to avoid phagocytosis;
inexpensive, readily
accessible and simple to
apply
PLA (Sculptra/Fill®) Biodegradable; usually Restoration of facial fat Assists with volume Volumization
derived from renewable loss (nasolabial folds, restoration while disappears within a
sources; injected into labiomental creases, simultaneously increasing few months; dermal
either the deep dermis or marionette lines, upper dermal thickness in the fibroplasia; Results not
subcutaneous layer. lip, etc.) face; reduced safety immediate (more than
concerns; very good 2 years)
risk-benefit profile for HIV
patients and for cosmetic
purposes
CaHA (Radiesse®) CaHA microspheres Correction of moderate-to- Biocompatible; skin Side effects more
acting as a scaffold for severe wrinkles and folds; testing is not required common; may be
new collagen formation volumization of facial soft- before use unsafe to use for
tissue;correction of the glabellar or periorbital
signs of facial lipoatrophy lines
Permanent fillers
Silicone (Silikon 1,000 and Oil injected as Volume and Low maintenance and Adverse complications;
AdatoSil 5,000) microdroplets contouring;injected cost; permanent cannot be removed
into the deep dermis or after implantation
subdermal plane
FDA: Food and Drug Administration; HA: hyaluronic acid; PMMA: poly(methyl methacrylate); PLA: poly-L-lactic acid; CaHA: calcium hydroxyapatite
study by Bailin and Bailin in which 8 patients were treated that the occurrence of local sensitive reaction ranges from
[8]
with Zyplast® and Zyderm® I and II for surgical scars, acne 3% to 5%. Various clinical tests and studies have shown that
scars and rhytids or a combination of these found that both Zyderm® and Zyplast®, if not used effectively, can cause
[9]
products were effective. Results lasted between 4-6 months adverse reactions. These reactions are often classified as
for rhytids and 6-9 months for scars regardless of which immune responses to the foreign material being injected
product was used. into the body. In general, Zyplast® and Zyderm® may
[9]
[8]
elicit adverse reactions such as hypersensitivity, herpes virus
Skin testing is essential for patients undergoing treatment reactivation, bacterial infection, bruising, and local necrosis.
with bovine collagen because it is extracted from an animal Therefore, it is crucial to follow strict procedures regarding
source and may provoke a reaction. Generally, two skin the skin testing protocol, as well as to compare it to other
tests spaced 2 or 4 weeks apart are recommended. The fillers to determine which one if appropriate for use.
skin test is performed by intradermal injection of 0.1 mL of
collagen onto the volar forearm to detect any pre-existing Porcine collagen
allergy to Zyderm® or its counterparts. It is suggested Porcine collagen is extracted from porcine tendon, dermis
[9]
74 Plast Aesthet Res || Vol 3 || Mar 23, 2016