Page 86 - Read Online
P. 86
Page 153 Millien et al. One Health Implement Res 2023;3:148-60 https://dx.doi.org/10.20517/ohir.2023.37
[18]
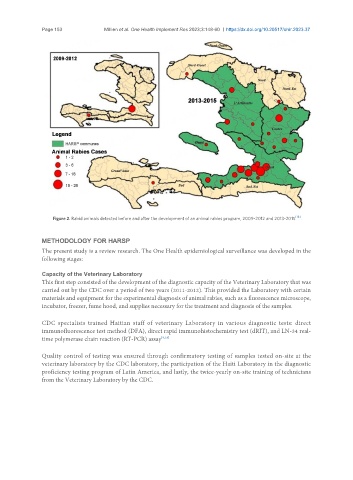
Figure 2. Rabid animals detected before and after the development of an animal rabies program, 2009-2012 and 2013-2015 .
METHODOLOGY FOR HARSP
The present study is a review research. The One Health epidemiological surveillance was developed in the
following stages:
Capacity of the Veterinary Laboratory
This first step consisted of the development of the diagnostic capacity of the Veterinary Laboratory that was
carried out by the CDC over a period of two years (2011-2012). This provided the Laboratory with certain
materials and equipment for the experimental diagnosis of animal rabies, such as a fluorescence microscope,
incubator, freezer, fume hood, and supplies necessary for the treatment and diagnosis of the samples.
CDC specialists trained Haitian staff of veterinary Laboratory in various diagnostic tests: direct
immunofluorescence test method (DFA), direct rapid immunohistochemistry test (dRIT), and LN-34 real-
time polymerase chain reaction (RT-PCR) assay [9,18]
Quality control of testing was ensured through confirmatory testing of samples tested on-site at the
veterinary laboratory by the CDC laboratory, the participation of the Haiti Laboratory in the diagnostic
proficiency testing program of Latin America, and lastly, the twice-yearly on-site training of technicians
from the Veterinary Laboratory by the CDC.