Page 26 - Read Online
P. 26
Page 204 Walker. Neuroimmunol Neuroinflammation 2020;7:194-214 I http://dx.doi.org/10.20517/2347-8659.2020.09
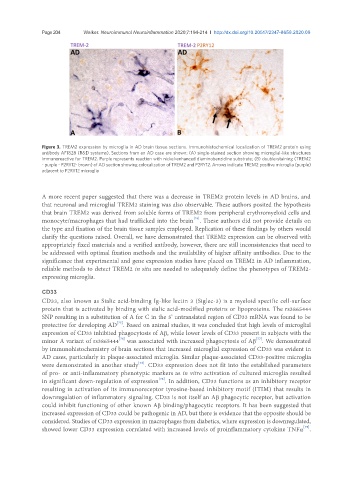
Figure 3. TREM2 expression by microglia in AD brain tissue sections. Immunohistochemical localization of TREM2 protein using
antibody AF1828 (R&D systems). Sections from an AD case are shown: (A) single-stained section showing microglial-like structures
immunoreactive for TREM2. Purple represents reaction with nickel-enhanced diaminobenzidine substrate; (B) double-staining (TREM2
- purple - P2RY12- brown) of AD section showing colocalization of TREM2 and P2RY12. Arrows indicate TREM2 positive microglia (purple)
adjacent to P2RY12 microglia
A more recent paper suggested that there was a decrease in TREM2 protein levels in AD brains, and
that neuronal and microglial TREM2 staining was also observable. These authors posited the hypothesis
that brain TREM2 was derived from soluble forms of TREM2 from peripheral erythromyeloid cells and
[74]
monocyte/macrophages that had trafficked into the brain . These authors did not provide details on
the type and fixation of the brain tissue samples employed. Replication of these findings by others would
clarify the questions raised. Overall, we have demonstrated that TREM2 expression can be observed with
appropriately fixed materials and a verified antibody, however, there are still inconsistencies that need to
be addressed with optimal fixation methods and the availability of higher affinity antibodies. Due to the
significance that experimental and gene expression studies have placed on TREM2 in AD inflammation,
reliable methods to detect TREM2 in situ are needed to adequately define the phenotypes of TREM2-
expressing microglia.
CD33
CD33, also known as Sialic acid-binding Ig-like lectin 3 (Siglec-3) is a myeloid specific cell-surface
protein that is activated by binding with sialic acid-modified proteins or lipoproteins. The rs3865444
SNP resulting in a substitution of A for C in the 5’ untranslated region of CD33 mRNA was found to be
[75]
protective for developing AD . Based on animal studies, it was concluded that high levels of microglial
expression of CD33 inhibited phagocytosis of Aβ, while lower levels of CD33 present in subjects with the
[77]
minor A variant of rs3865444 was associated with increased phagocytosis of Aβ . We demonstrated
[76]
by immunohistochemistry of brain sections that increased microglial expression of CD33 was evident in
AD cases, particularly in plaque-associated microglia. Similar plaque-associated CD33-positive microglia
[78]
were demonstrated in another study . CD33 expression does not fit into the established parameters
of pro- or anti-inflammatory phenotypic markers as in vitro activation of cultured microglia resulted
[76]
in significant down-regulation of expression . In addition, CD33 functions as an inhibitory receptor
resulting in activation of its immunoreceptor tyrosine-based inhibitory motif (ITIM) that results in
downregulation of inflammatory signaling. CD33 is not itself an Aβ phagocytic receptor, but activation
could inhibit functioning of other known Aβ binding/phagocytic receptors. It has been suggested that
increased expression of CD33 could be pathogenic in AD, but there is evidence that the opposite should be
considered. Studies of CD33 expression in macrophages from diabetics, where expression is downregulated,
[79]
showed lower CD33 expression correlated with increased levels of proinflammatory cytokine TNFα .