Page 89 - Read Online
P. 89
Harry et al. Neuroimmunol Neuroinflammation 2020;7:150-65 I http://dx.doi.org/10.20517/2347-8659.2020.07 Page 157
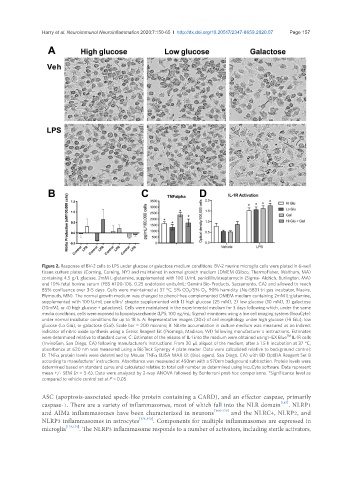
Figure 2. Response of BV-2 cells to LPS under glucose or galactose medium conditions. BV-2 murine microglia cells were plated in 6-well
tissue culture plates (Corning, Corning, NY) and maintained in normal growth medium (DMEM (Gibco, ThermoFisher, Waltham, MA)
containing 4.5 g/L glucose, 2mM L-glutamine, supplemented with 100 U/mL penicillin/streptomycin (Sigma- Aldrich, Burlington, MA)
and 10% fetal bovine serum (FBS #100-106, 0.25 endotoxin units/mL; Gemini Bio-Products, Sacramento, CA) and allowed to reach
85% confluence over 3-5 days. Cells were maintained at 37 °C, 5% CO 2 /5% O 2 , 90% humidity (Nu-5831 tri gas incubator, Nuaire,
Plymouth, MN). The normal growth medium was changed to phenol-free complemented DMEM medium containing 2mM L-glutamine,
supplemented with 100 U/mL penicllin/ strepto supplemented with 1) high glucose (25 mM), 2) low glucose (10 mM), 3) galactose
(10mM), or 4) high glucose + galactose). Cells were maintained in the experimental medium for 3 days following which, under the same
media conditions, cells were exposed to lipopolysaccharide (LPS; 100 ng/mL; Sigma) monitorec using a live cell imaging system (IncuCyte)
under normal incubator conditions for up to 18 h. A: Representative images (20x) of cell morphology under high glucose (Hi Glu), low
glucose (Lo Glu), or galactose (Gal). Scale bar = 200 microns; B: Nitrite accumulation in culture medium was measured as an indirect
indicator of nitric oxide synthesis using a Greiss Reagent kit (Promega, Madison, WI) following manufacturer s instructions. Estimates
TM
were determined relative to standard curve; C: Estimates of the release of IL-1 into the medium were obtained using HEX Blue IL-1R cells
(InvivoGen, San Diego, CA) following manufacturer’s instructions. From 20 μL aliquot of the medium, after a 1.5 h incubation at 37 °C,
absorbance at 620 nm was measured using a BioTeck Synergy 4 plate reader. Data were calculated relative to background control;
D: TNFa protein levels were determined by Mouse TNFa ELISA MAX kit (BioL egend, San Diego, CA) with BD OptEIA Reagent Set B
according to manufacturer’ instructions. Absorbance was measured at 450nm with a 570nm background subtraction. Protein levels were
determined based on standard curve and calculated relative to total cell number as determined using IncuCyte software. Data represent
mean +/- SEM (n = 5-6). Data were analyzed by 2-way ANOVA followed by Bonferroni post-hoc comparisons. *Significance level as
compared to vehicle control set at P < 0.05
ASC (apoptosis-associated speck-like protein containing a CARD), and an effector caspase, primarily
caspase-1. There are a variety of inflammasomes, most of which fall into the NLR domain [167] . NLRP1
and AIM2 inflammasomes have been characterized in neurons [168-170] and the NLRC4, NLRP2, and
NLRP3 inflammasomes in astrocytes [171,172] . Components for multiple inflammasomes are expressed in
microglia [173,174] . The NLRP3 inflammasome responds to a number of activators, including sterile activators,