Page 78 - Read Online
P. 78
Page 146 Das et al. Neuroimmunol Neuroinflammation 2020;7:141-9 I http://dx.doi.org/10.20517/2347-8659.2020.36
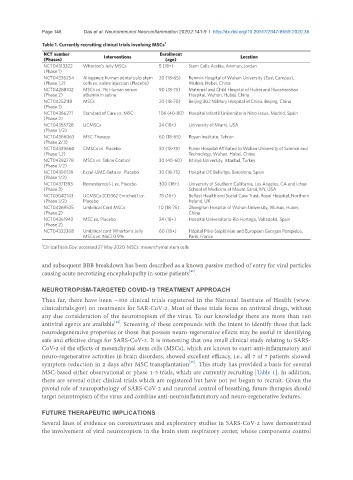
Table 1. Currently recruiting clinical trials involving MSCs 1
NCT number Enrollment
(Phases) Interventions (age) Location
NCT04313322 Wharton’s Jelly MSCs 5 (18+) Stem Cells Arabia, Amman, Jordan
(Phase 1)
NCT04336254 Allogeneic human dental pulp stem 20 (18-65) Renmin Hospital of Wuhan University (East Campus),
(Phase 1,2) cells vs. saline injection (Placebo) Wuhan, Hubei, China
NCT04288102 MSCs vs. 1% Human serum 90 (18-75) Maternal and Child Hospital of Hubei and Huoshenshan
(Phase 2) albumin in saline Hospital, Wuhan, Hubei, China
NCT04252118 MSCs 20 (18-70) Beijing 302 Military Hospital of China, Beijing, China
(Phase 1)
NCT04366271 Standard of Care vs. MSC 106 (40-80) Hospital Infantil Universitario Nino Jesus, Madrid, Spain
(Phase 2)
NCT04355728 UCMSCs 24 (18+) University of Miami, USA
(Phase 1/2)
NCT04366063 MSC Therapy 60 (18-65) Royan Institute, Tehran
(Phase 2/3)
NCT04339660 CMSCs vs. Placebo 30 (18-75) Puren Hospital Affiliated to Wuhan University of Science and
(Phase 1,2) Technology, Wuhan, Hubei, China
NCT04392778 MSCs vs. Saline Control 30 (40-60) Istinye University, Istanbul, Turkey
(Phase 1/2)
NCT04390139 Excel-UMC-Beta vs. Placebo 30 (18-75) Hospital DE Bellvitge, Barcelona, Spain
(Phase 1/2)
NCT04371393 Remestemcel-L vs. Placebo 300 (18+) University of Southern California, Los Angeles, CA and Ichan
(Phase 3) School of Medicine at Mount Sanai, NY, USA
NCT03042143 UCMSCs (CD362 Enriched) vs. 75 (16+) Belfast Health and Social Care Trust, Royal Hospital, Northern
(Phase 1/2) Placebo Ireland, UK
NCT04269525 Umbilical Cord MSCs 10 (18-75) Zhongnan Hospital of Wuhan University, Wuhan, Hubei,
(Phase 2) China
NCT04361942 MSC vs. Placebo 24 (18+) Hospital Universitario Rio Hortega, Valladolid, Spain
(Phase 2)
NCT04333368 Umbilical cord Wharton’s Jelly 60 (18+) Hôpital Pitié-Salpêtrière and Européen Georges Pompidou,
MSCs vs. NaCl 0.9% Paris, France
1 ClinicalTrials.Gov; accessed 27 May 2020. MSCs: mesenchymal stem cells
and subsequent BBB breakdown has been described as a known passive method of entry for viral particles
causing acute necrotizing encephalopathy in some patients .
[43]
NEUROTROPISM-TARGETED COVID-19 TREATMENT APPROACH
Thus far, there have been ~300 clinical trials registered in the National Institute of Health (www.
clinicaltrials.gov) on treatments for SAR-CoV-2. Most of these trials focus on antiviral drugs, without
any due consideration of the neurotropism of the virus. To our knowledge there are more than 160
[44]
antiviral agents are available . Screening of these compounds with the intent to identify those that lack
neurodegenerative properties or those that possess neuro-regenerative effects may be useful in identifying
safe and effective drugs for SARS-CoV-2. It is interesting that one small clinical study relating to SARS-
CoV-2 of the effects of mesenchymal stem cells (MSCs), which are known to exert anti-inflammatory and
neuro-regenerative activities in brain disorders, showed excellent efficacy, i.e., all 7 of 7 patients showed
[45]
symptom reduction in 2 days after MSC transplantation . This study has provided a basis for several
MSC-based either observational or phase 1-3 trials, which are currently recruiting [Table 1]. In addition,
there are several other clinical trials which are registered but have not yet begun to recruit. Given the
pivotal role of neuropathology of SARS-CoV-2 and neuronal control of breathing, future therapies should
target neurotropism of the virus and combine anti-neuroinflammatory and neuro-regenerative features.
FUTURE THERAPEUTIC IMPLICATIONS
Several lines of evidence on coronaviruses and exploratory studies in SARS-CoV-2 have demonstrated
the involvement of viral neurotropism in the brain stem respiratory center, whose components control