Page 59 - Read Online
P. 59
Page 8 of 16 Zhang et al. Neuroimmunol Neuroinflammation 2019;6:8 I http://dx.doi.org/10.20517/2347-8659.2019.06
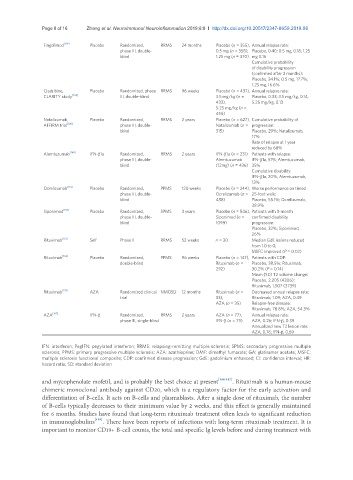
Fingolimod [119] Placebo Randomized, RRMS 24 months Placebo (n = 355), Annual relapse rate:
phase III, double- 0.5 mg (n = 358), Placebo, 0.40; 0.5 mg, 0.18; 1.25
blind 1.25 mg (n = 370) mg 0.16
Cumulative probability
of disability progression
(confirmed after 3 months):
Placebo, 24.1%; 0.5 mg, 17.7%;
1.25 mg, 16.6%
Cladribine, Placebo Randomized, phase RRMS 96 weeks Placebo (n = 437), Annual relapse rate:
CLARITY study [154] III, double-blind 3.5 mg/kg (n = Placebo, 0.33; 3.5 mg/kg, 0.14;
433), 5.25 mg/kg, 0.13
5.25 mg/kg (n =
456)
Natalizumab, Placebo Randomized, RRMS 2 years Placebo (n = 627), Cumulative probability of
AFFIRM trial [140] phase III, double- Natalizumab (n = progression:
blind 315) Placebo, 29%; Natalizumab,
17%
Rate of relapse at 1 year
reduced by 68%
Alemtuzumab [148] IFN-β1a Randomized, RRMS 2 years IFN-β1a (n = 231) Patients with relapse:
phase III, double- Alemtuzumab IFN-β1a, 51%; Alemtuzumab,
blind (12mg) (n = 436) 35%
Cumulative disability:
IFN-β1a, 20%; Alemtuzumab,
13%
Ocrelizumab [151] Placebo Randomized, PPMS 120 weeks Placebo (n = 244), Worse performance on timed
phase III, double- Ocrelizumab (n = 25-foot walk:
blind 488) Placebo, 55.1%; Ocrelizumab,
38.9%
Siponimod [156] Placebo Randomized, SPMS 3 years Placebo (n = 546), Patients with 3-month
phase III, double- Siponimod (n = confirmed disability
blind 1099) progression:
Placebo, 32%; Siponimod,
26%
Rituximab [161] Self Phase II RRMS 52 weeks n = 30 Median GdE lesions reduced
from 1.0 to 0;
MSFC improved (P = 0.02)
Rituximab [162] Placebo Randomized, PPMS 96 weeks Placebo (n = 147), Patients with CDP:
double-blind Rituximab (n = Placebo, 38.5%; Rituximab,
292) 30.2% (P = 0.14)
Mean (SD) T2 volume change:
Placebo, 2,205 (4306);
Rituximab, 1,507 (3739)
Rituximab [159] AZA Randomized clinical NMOSD 12 months Rituximab (n = Decreased annual relapse rate:
trial 33), Rituximab, 1.09; AZA, 0.49
AZA (n = 35) Relapse-free disease:
Rituximab, 78.8%; AZA, 54.3%
AZA [167] IFN-β Randomized, RRMS 2 years AZA (n = 77), Annual relapse rate:
phase III, single-blind IFN-β (n = 73) AZA, 0.26; IFN-β, 0.39
Annualized new T2 lesion rate:
AZA, 0.76; IFN-β, 0.69
IFN: interferon; PegIFN: pegylated interferon; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple
sclerosis; PPMS: primary progressive multiple sclerosis; AZA: azathioprine; DMF: dimethyl fumarate; GA: glatiramer acetate; MSFC:
multiple sclerosis functional composite; CDP: confirmed disease progression; GdE: gadolinium enhanced; CI: confidence interval; HR:
hazard ratio; SD: standard deviation
and mycophenolate mofetil, and is probably the best choice at present [184-187] . Rituximab is a human-mouse
chimeric monoclonal antibody against CD20, which is a regulatory factor for the early activation and
differentiation of B-cells. It acts on B-cells and plasmablasts. After a single dose of rituximab, the number
of B-cells typically decreases to their minimum value by 2 weeks, and this effect is generally maintained
for 6 months. Studies have found that long-term rituximab treatment often leads to significant reduction
in immunoglobulins [188] . There have been reports of infections with long-term rituximab treatment. It is
important to monitor CD19+ B-cell counts, the total and specific Ig levels before and during treatment with