Page 58 - Read Online
P. 58
Zhang et al. Neuroimmunol Neuroinflammation 2019;6:8 I http://dx.doi.org/10.20517/2347-8659.2019.06 Page 7 of 16
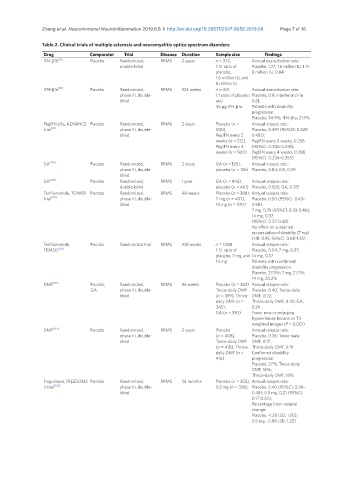
Table 2. Clinical trials of multiple sclerosis and neuromyelitis optica spectrum disorders
Drug Comparator Trial Disease Duration Sample size Findings
IFN-β1b [87] Placebo Randomized, RRMS 2 years n = 372, Annual exacerbation rate:
double-blind 1:1:1 ratio of Placebo, 1.27; 1.6 million IU, 1.17;
placebo, 8 million IU, 0.84
1.6 million IU, and
8 million IU
IFN-β1a [89] Placebo Randomized, RRMS 104 weeks n = 301, Annual exacerbation rate:
phase III, double- 1:1 ratio of placebo Placebo, 0.9; interferon β-1a
blind and 0.61;
30 μg IFN-β1a Patients with disability
progression:
Placebo, 34.9%; IFN-β1a, 21.9%
PegIFN-β1a, ADVANCE Placebo Randomized, RRMS 2 years Placebo (n = Annual relapse rate:
trial [93] phase III, double- 500), Placebo, 0.397 (95%CI: 0.328-
blind PegIFN every 2 0.481);
weeks (n = 512), PegIFN every 2 weeks, 0.256
PegIFN every 4 (95%CI: 0.206-0.318);
weeks (n = 500) PegIFN every 4 weeks, 0.288
(95%CI: 0.234-0.355)
GA [105] Placebo Randomized, RRMS 2 years GA (n = 125), Annual relapse rate:
phase III, double- placebo (n = 126) Placebo, 0.84; GA, 0.59
blind
GA [109] Placebo Randomized, RRMS 1 year GA (n = 943), Annual relapse rate:
double-blind placebo (n = 461) Placebo, 0.505; GA, 0.331
Teriflunomide, TOWER Placebo Randomized, RRMS 48 weeks Placebo (n = 388), Annual relapse rate:
trial [128] phase III, double- 7 mg (n = 407), Placebo, 0.50 (95%CI: 0.43-
blind 14 mg (n = 370) 0.58);
7 mg, 0.39 (95%CI: 0.33-0.46);
14 mg, 0.32
(95%CI: 0.27-0.38)
No effect on sustained
accumulation of disability (7 mg)
(HR: 0.95, 95%CI: 0.68-1.35)
Teriflunomide, Placebo Randomized trial RRMS 108 weeks n = 1088 Annual relapse rate:
TEMSO [129] 1:1:1 ratio of Placebo, 0.54; 7 mg, 0.37;
placebo, 7 mg, and 14 mg, 0.37
14 mg Patients with confirmed
disability progression:
Placebo, 27.3%; 7 mg, 21.7%;
14 mg, 20.2%
DMF [135] Placebo, Randomized, RRMS 96 weeks Placebo (n = 363) Annual relapse rate:
GA phase III, double- Twice-daily DMF Placebo, 0.40; Twice-daily
blind (n = 359), Thrice- DMF, 0.22;
daily DMF (n = Thrice-daily DMF, 0.20; GA,
345), 0.29
GA (n = 350) Fewer new or enlarging
hyperintense lesions on T2-
weighted images (P < 0.001)
DMF [134] Placebo Randomized, RRMS 2 years Placebo Annual relapse rate:
phase III, double- (n = 408), Placebo, 0.36; Twice-daily
blind Twice-daily DMF DMF, 0.17;
(n = 410), Thrice- Thrice-daily DMF, 0.19
daily DMF (n = Confirmed disability
416) progression:
Placebo, 27%; Twice-daily
DMF, 16%;
Thrice-daily DMF, 18%
Fingolimod, FREEDOMS Placebo Randomized, RRMS 24 months Placebo (n = 355), Annual relapse rate:
II trial [120] phase III, double- 0.5 mg (n = 358), Placebo, 0.40 (95%CI: 0.34-
blind 0.48); 0.5 mg, 0.21 (95%CI:
0.17-0.25);
Percentage brain volume
change:
Placebo, -1.28 (SD, 1.50);
0.5 mg, -0.86 (SD, 1.22)