Page 69 - Read Online
P. 69
Page 4 of 8 Li et al. Metab Target Organ Damage. 2025;5:19 https://dx.doi.org/10.20517/mtod.2025.05
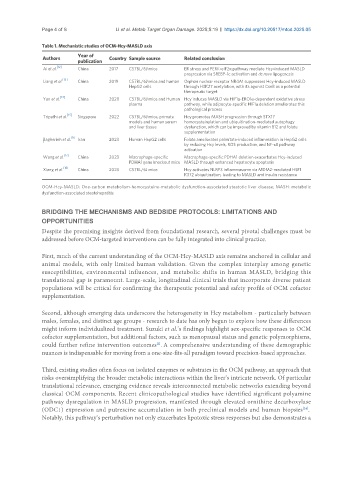
Table 1. Mechanistic studies of OCM-Hcy-MASLD axis
Year of
Authors Country Sample source Related conclusion
publication
[12]
Ai et al. China 2017 C57BL/6J mice ER stress and PERK-eIF2α pathway mediate Hcy-induced MASLD
progression via SREBP-1c activation and de novo lipogenesis
[13]
Liang et al. China 2019 C57BL/6J mice and human Orphan nuclear receptor NR4A1 suppresses Hcy-induced MASLD
HepG2 cells through H3K27 acetylation, with its agonist CsnB as a potential
therapeutic target
[14]
Yan et al. China 2020 C57BL/6J mice and Human Hcy induces MASLD via HIF1α-ERO1α-dependent oxidative stress
plasma pathway, while adipocyte-specific HIF1α deletion ameliorates this
pathological process
[15]
Tripathi et al. Singapore 2022 C57BL/6J mice, primate Hcy promotes MASH progression through STX17
models and human serum homocysteinylation and ubiquitination-mediated autophagy
and liver tissue dysfunction, which can be improved by vitamin B12 and folate
supplementation
[16
Bagherieh et al. Iran 2023 Human HepG2 cells Folate ameliorates palmitate-induced inflammation in HepG2 cells
]
by reducing Hcy levels, ROS production, and NF-κB pathway
activation
Wang et al. [17] China 2023 Macrophage-specific Macrophage-specific PDHA1 deletion exacerbates Hcy-induced
PDHA1 gene knockout mice MASLD through enhanced hepatocyte apoptosis
[18]
Xiang et al. China 2023 C57BL/6J mice Hcy activates NLRP3 inflammasome via MDM2-mediated HSF1
K372 ubiquitination, leading to MASLD and insulin resistance
OCM-Hcy-MASLD: One-carbon metabolism-homocysteine-metabolic dysfunction-associated steatotic liver disease; MASH: metabolic
dysfunction-associated steatohepatitis.
BRIDGING THE MECHANISMS AND BEDSIDE PROTOCOLS: LIMITATIONS AND
OPPORTUNITIES
Despite the promising insights derived from foundational research, several pivotal challenges must be
addressed before OCM-targeted interventions can be fully integrated into clinical practice.
First, much of the current understanding of the OCM-Hcy-MASLD axis remains anchored in cellular and
animal models, with only limited human validation. Given the complex interplay among genetic
susceptibilities, environmental influences, and metabolic shifts in human MASLD, bridging this
translational gap is paramount. Large-scale, longitudinal clinical trials that incorporate diverse patient
populations will be critical for confirming the therapeutic potential and safety profile of OCM cofactor
supplementation.
Second, although emerging data underscore the heterogeneity in Hcy metabolism - particularly between
males, females, and distinct age groups - research to date has only begun to explore how these differences
might inform individualized treatment. Suzuki et al.’s findings highlight sex-specific responses to OCM
cofactor supplementation, but additional factors, such as menopausal status and genetic polymorphisms,
[2]
could further refine intervention outcomes . A comprehensive understanding of these demographic
nuances is indispensable for moving from a one-size-fits-all paradigm toward precision-based approaches.
Third, existing studies often focus on isolated enzymes or substrates in the OCM pathway, an approach that
risks oversimplifying the broader metabolic interactions within the liver’s intricate network. Of particular
translational relevance, emerging evidence reveals interconnected metabolic networks extending beyond
classical OCM components. Recent clinicopathological studies have identified significant polyamine
pathway dysregulation in MASLD progression, manifested through elevated ornithine decarboxylase
[24]
(ODC1) expression and putrescine accumulation in both preclinical models and human biopsies .
Notably, this pathway’s perturbation not only exacerbates lipotoxic stress responses but also demonstrates a