Page 595 - Read Online
P. 595
Page 4 of 15 Saxena et al. Mini-invasive Surg 2020;4:62 I http://dx.doi.org/10.20517/2574-1225.2020.68
Figure 1. Chemical structure of chitosan, comprising N-acetyl-D-glucosamine (right) and D-glucosamine (left) units. Adapted with
permission from Andrade et al. [33]
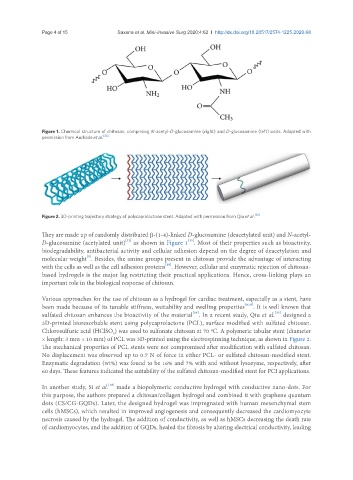
Figure 2. 3D-printing trajectory strategy of polycaprolactone stent. Adapted with permission from Qiu et al. [35]
They are made up of randomly distributed β-(1-4)-linked D-glucosamine (deacetylated unit) and N-acetyl-
[33]
[32]
D-glucosamine (acetylated unit) as shown in Figure 1 . Most of their properties such as bioactivity,
biodegradability, antibacterial activity and cellular adhesion depend on the degree of deacetylation and
[9]
molecular weight . Besides, the amine groups present in chitosan provide the advantage of interacting
[18]
with the cells as well as the cell adhesion proteins . However, cellular and enzymatic rejection of chitosan-
based hydrogels is the major lag restricting their practical applications. Hence, cross-linking plays an
important role in the biological response of chitosan.
Various approaches for the use of chitosan as a hydrogel for cardiac treatment, especially as a stent, have
been made because of its tunable stiffness, wettability and swelling properties [9,18] . It is well known that
sulfated chitosan enhances the bioactivity of the material . In a recent study, Qiu et al. designed a
[34]
[35]
3D-printed bioresorbable stent using polycaprolactone (PCL), surface modified with sulfated chitosan.
Chlorosulfuric acid (HClSO ) was used to sulfonate chitosan at 70 ºC. A polymeric tabular stent (diameter
3
× length: 3 mm × 10 mm) of PCL was 3D-printed using the electrospinning technique, as shown in Figure 2.
The mechanical properties of PCL stents were not compromised after modification with sulfated chitosan.
No displacement was observed up to 0.7 N of force in either PCL- or sulfated chitosan-modified stent.
Enzymatic degradation (wt%) was found to be 16% and 7% with and without lysozyme, respectively, after
60 days. These features indicated the suitability of the sulfated chitosan-modified stent for PCI applications.
[18]
In another study, Si et al. made a biopolymeric conductive hydrogel with conductive nano-dots. For
this purpose, the authors prepared a chitosan/collagen hydrogel and combined it with graphene quantum
dots (CS/CG-GQDs). Later, the designed hydrogel was impregnated with human mesenchymal stem
cells (hMSCs), which resulted in improved angiogenesis and consequently decreased the cardiomyocyte
necrosis caused by the hydrogel. The addition of conductivity, as well as hMSCs decreasing the death rate
of cardiomyocytes, and the addition of GQDs, healed the fibrosis by altering electrical conductivity, leading