Page 26 - Read Online
P. 26
Sawabata et al. Pulmonary wedge resection for NSCLC
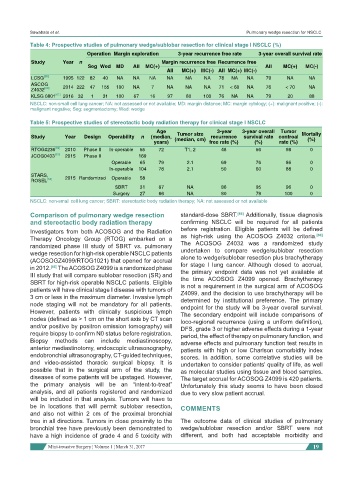
Table 4: Prospective studies of pulmonary wedge/sublobar resection for clinical stage I NSCLC (%)
Operation Margin exploration 3-year recurrence free rate 3-year overall survival rate
Study Year n Margin recurrence free Recurrence free
Seg Wed MD All MC(+) All MC(+) MC(-)
All MC(+) MC(-) All MC(+) MC(-)
LCSG [55] 1995 122 82 40 NA NA NA NA NA NA 78 NA NA 79 NA NA
ASCOG
Z4032 [60] 2014 222 47 155 100 NA 7 NA NA NA 71 < 60 NA 76 < 70 NA
KLSG 0801 [61] 2016 32 1 31 100 67 16 97 80 100 76 NA NA 79 20 88
NSCLC: non-small cell lung cancer; NA: not assessed or not available; MD: margin distance; MC: margin cytology; (+): malignant positive; (-):
malignant negative; Seg: segmentectomy; Wed: wedge
Table 5: Prospective studies of stereotactic body radiation therapy for clinical stage I NSCLC
Age Tumor size 3-year 3-year overall Tumor Mortaliy
Study Year Design Operability n (median, (median, cm) recurrence survival rate controal (%)
years) free rate (%) (%) rate (%)
RTOG0236 [62] 2010 Phase II In-operable 55 72 T1, 2 48 56 98 0
JCOG0403 [63] 2015 Phase II 169
Operable 65 79 2.1 69 76 86 0
In-operable 104 78 2.1 50 60 88 0
STARS,
ROSEL [64] 2015 Randomized Operable 58
SBRT 31 67 NA 86 95 96 0
Surgery 27 66 NA 80 79 100 0
NSCLC: non-small cell lung cancer; SBRT: stereotactic body radiation therapy; NA: not assessed or not available
Comparison of pulmonary wedge resection standard-dose SBRT. [66] Additionally, tissue diagnosis
and stereotactic body radiation therapy confirming NSCLC will be required for all patients
Investigators from both ACOSOG and the Radiation before registration. Eligible patients will be defined
Therapy Oncology Group (RTOG) embarked on a as high-risk using the ACOSOG Z4032 criteria. [60]
randomized phase III study of SBRT vs. pulmonary The ACOSOG Z4032 was a randomized study
wedge resection for high-risk operable NSCLC patients undertaken to compare wedge/sublobar resection
(ACOSOGZ4099/RTOG1021) that opened for accrual alone to wedge/sublobar resection plus brachytherapy
in 2012. [65] The ACOSOG Z4099 is a randomized phase for stage I lung cancer. Although closed to accrual,
III study that will compare sublobar resection (SR) and the primary endpoint data was not yet available at
the time ACOSOG Z4099 opened. Brachytherapy
SBRT for high-risk operable NSCLC patients. Eligible is not a requirement in the surgical arm of ACOSOG
patients will have clinical stage I disease with tumors of Z4099, and the decision to use brachytherapy will be
3 cm or less in the maximum diameter. Invasive lymph determined by institutional preference. The primary
node staging will not be mandatory for all patients. endpoint for the study will be 3-year overall survival.
However, patients with clinically suspicious lymph The secondary endpoint will include comparisons of
nodes (defined as > 1 cm on the short axis by CT scan loco-regional recurrence (using a uniform definition),
and/or positive by positron emission tomography) will DFS, grade 3 or higher adverse effects during a 1-year
require biopsy to confirm N0 status before registration. period, the effect of therapy on pulmonary function, and
Biopsy methods can include mediastinoscopy, adverse effects and pulmonary function test results in
anterior mediastinotomy, endoscopic ultrasonography, patients with high or low Charlson comorbidity index
endobronchial ultrasonography, CT-guided techniques, scores. In addition, some correlative studies will be
and video-assisted thoracic surgical biopsy. It is undertaken to consider patients’ quality of life, as well
possible that in the surgical arm of the study, the as molecular studies using tissue and blood samples.
diseases of some patients will be upstaged. However, The target accrual for ACOSOG Z4099 is 420 patients.
the primary analysis will be an “intent-to-treat” Unfortunately this study seems to have been closed
analysis, and all patients registered and randomized due to very slow patient accrual.
will be included in that analysis. Tumors will have to
be in locations that will permit sublobar resection, COMMENTS
and also not within 2 cm of the proximal bronchial
tree in all directions. Tumors in close proximity to the The outcome data of clinical studies of pulmonary
bronchial tree have previously been demonstrated to wedge/sublobar resection and/or SBRT were not
have a high incidence of grade 4 and 5 toxicity with different, and both had acceptable morbidity and
Mini-invasive Surgery ¦ Volume 1 ¦ March 31, 2017 19