Page 47 - Read Online
P. 47
Page 10 of 14 Abe et al. Mini-invasive Surg 2023;7:28 https://dx.doi.org/10.20517/2574-1225.2023.15
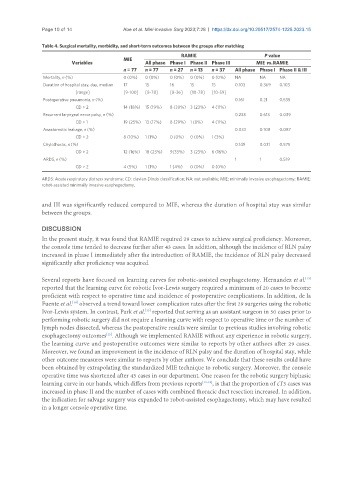
Table 4. Surgical mortality, morbidity, and short-term outcomes between the groups after matching
RAMIE P value
MIE
Variables All phase Phase I Phase II Phase III MIE vs. RAMIE
n = 77 n = 77 n = 27 n = 13 n = 37 All phase Phase I Phase II & III
Mortality, n (%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) NA NA NA
Duration of hospital stay, day, median 17 15 16 15 15 0.103 0.369 0.103
[range] [9-100] [8-78] [8-36] [10-78] [10-59]
Postoperative pneumonia, n (%) 0.161 0.21 0.535
CD > 2 14 (18%) 15 (19%) 8 (30%) 3 (23%) 4 (11%)
Recurrent laryngeal nerve palsy, n (%) 0.233 0.613 0.039
CD > 1 19 (25%) 13 (17%) 8 (29%) 1 (8%) 4 (11%)
Anastomotic leakage, n (%) 0.033 0.108 0.087
CD > 2 8 (10%) 1 (1%) 0 (0%) 0 (0%) 1 (3%)
Chylothorax, n (%) 0.149 0.031 0.575
CD > 2 12 (16%) 18 (23%) 9(33%) 3 (23%) 6 (16%)
ARDS, n (%) 1 1 0.519
CD > 2 4 (5%) 1 (1%) 1 (4%) 0 (0%) 0 (0%)
ARDS: Acute respiratory distress syndrome; CD: clavien-Dindo classification; NA: not available; MIE: minimally invasive esophagectomy; RAMIE:
robot-assisted minimally invasive esophagectomy.
and III was significantly reduced compared to MIE, whereas the duration of hospital stay was similar
between the groups.
DISCUSSION
In the present study, it was found that RAMIE required 29 cases to achieve surgical proficiency. Moreover,
the console time tended to decrease further after 43 cases. In addition, although the incidence of RLN palsy
increased in phase I immediately after the introduction of RAMIE, the incidence of RLN palsy decreased
significantly after proficiency was acquired.
Several reports have focused on learning curves for robotic-assisted esophagectomy. Hernandez et al.
[13]
reported that the learning curve for robotic Ivor-Lewis surgery required a minimum of 20 cases to become
proficient with respect to operative time and incidence of postoperative complications. In addition, de la
Fuente et al. observed a trend toward lower complication rates after the first 29 surgeries using the robotic
[14]
Ivor-Lewis system. In contrast, Park et al. reported that serving as an assistant surgeon in 50 cases prior to
[15]
performing robotic surgery did not require a learning curve with respect to operative time or the number of
lymph nodes dissected, whereas the postoperative results were similar to previous studies involving robotic
esophagectomy outcomes . Although we implemented RAMIE without any experience in robotic surgery,
[15]
the learning curve and postoperative outcomes were similar to reports by other authors after 29 cases.
Moreover, we found an improvement in the incidence of RLN palsy and the duration of hospital stay, while
other outcome measures were similar to reports by other authors. We conclude that these results could have
been obtained by extrapolating the standardized MIE technique to robotic surgery. Moreover, the console
operative time was shortened after 43 cases in our department. One reason for the robotic surgery biphasic
learning curve in our hands, which differs from previous reports [13-15] , is that the proportion of cT3 cases was
increased in phase II and the number of cases with combined thoracic duct resection increased. In addition,
the indication for salvage surgery was expanded to robot-assisted esophagectomy, which may have resulted
in a longer console operative time.