Page 39 - Read Online
P. 39
Nwaiwu et al. Mini-invasive Surg. 2025;9:20 https://dx.doi.org/10.20517/2574-1225.2024.112 Page 13 of 20
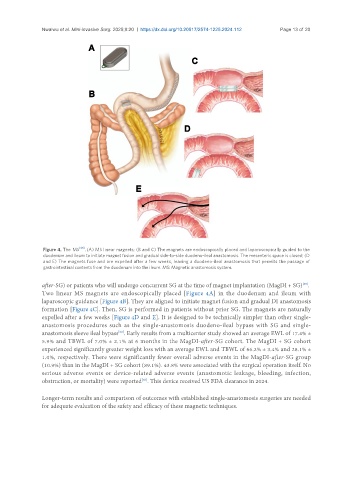
Figure 4. The MS [66] . (A) MS linear magnets; (B and C) The magnets are endoscopically placed and laparoscopically guided to the
duodenum and ileum to initiate magnet fusion and gradual side-to-side duodeno-ileal anastomosis. The mesenteric space is closed; (D
and E) The magnets fuse and are expelled after a few weeks, leaving a duodeno-ileal anastomosis that permits the passage of
gastrointestinal contents from the duodenum into the ileum. MS: Magnetic anastomosis system.
[66]
after-SG) or patients who will undergo concurrent SG at the time of magnet implantation (MagDI + SG) .
Two linear MS magnets are endoscopically placed [Figure 4A] in the duodenum and ileum with
laparoscopic guidance [Figure 4B]. They are aligned to initiate magnet fusion and gradual DI anastomosis
formation [Figure 4C]. Then, SG is performed in patients without prior SG. The magnets are naturally
expelled after a few weeks [Figure 4D and E]. It is designed to be technically simpler than other single-
anastomosis procedures such as the single-anastomosis duodeno-ileal bypass with SG and single-
anastomosis sleeve ileal bypass . Early results from a multicenter study showed an average EWL of 17.4% ±
[66]
5.9% and TBWL of 7.0% ± 2.1% at 6 months in the MagDI-after-SG cohort. The MagDI + SG cohort
experienced significantly greater weight loss with an average EWL and TBWL of 66.2% ± 3.4% and 28.1% ±
1.0%, respectively. There were significantly fewer overall adverse events in the MagDI-after-SG group
(10.9%) than in the MagDI + SG cohort (89.1%). 43.8% were associated with the surgical operation itself. No
serious adverse events or device-related adverse events (anastomotic leakage, bleeding, infection,
[66]
obstruction, or mortality) were reported . This device received US FDA clearance in 2024.
Longer-term results and comparison of outcomes with established single-anastomosis surgeries are needed
for adequate evaluation of the safety and efficacy of these magnetic techniques.