Page 34 - Read Online
P. 34
Page 8 of 20 Nwaiwu et al. Mini-invasive Surg. 2025;9:20 https://dx.doi.org/10.20517/2574-1225.2024.112
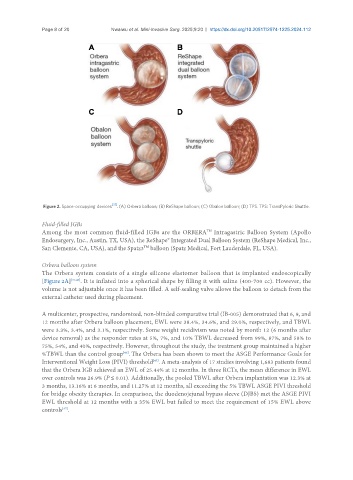
Figure 2. Space-occupying devices [37] . (A) Orbera balloon; (B) ReShape balloon; (C) Obalon balloon; (D) TPS. TPS: TransPyloric Shuttle.
Fluid-filled IGBs
Among the most common fluid-filled IGBs are the ORBERA Intragastric Balloon System (Apollo
TM
Endosurgery, Inc., Austin, TX, USA), the ReShape® Integrated Dual Balloon System (ReShape Medical, Inc.,
San Clemente, CA, USA), and the Spatz3 balloon (Spatz Medical, Fort Lauderdale, FL, USA).
TM
Orbera balloon system
The Orbera system consists of a single silicone elastomer balloon that is implanted endoscopically
[Figure 2A] [37,46] . It is inflated into a spherical shape by filling it with saline (400-700 cc). However, the
volume is not adjustable once it has been filled. A self-sealing valve allows the balloon to detach from the
external catheter used during placement.
A multicenter, prospective, randomized, non-blinded comparative trial (IB-005) demonstrated that 6, 9, and
12 months after Orbera balloon placement, EWL were 38.4%, 34.6%, and 29.0%, respectively, and TBWL
were 3.3%, 3.4%, and 3.1%, respectively. Some weight recidivism was noted by month 12 (6 months after
device removal) as the responder rates at 5%, 7%, and 10% TBWL decreased from 99%, 87%, and 58% to
75%, 54%, and 40%, respectively. However, throughout the study, the treatment group maintained a higher
%TBWL than the control group . The Orbera has been shown to meet the ASGE Performance Goals for
[46]
Interventional Weight Loss (PIVI) threshold . A meta-analysis of 17 studies involving 1,683 patients found
[47]
that the Orbera IGB achieved an EWL of 25.44% at 12 months. In three RCTs, the mean difference in EWL
over controls was 26.9% (P ≤ 0.01). Additionally, the pooled TBWL after Orbera implantation was 12.3% at
3 months, 13.16% at 6 months, and 11.27% at 12 months, all exceeding the 5% TBWL ASGE PIVI threshold
for bridge obesity therapies. In comparison, the duodenojejunal bypass sleeve (DJBS) met the ASGE PIVI
EWL threshold at 12 months with a 35% EWL but failed to meet the requirement of 15% EWL above
controls .
[47]