Page 90 - Read Online
P. 90
Chang et al. Mini-invasive Surg 2024;8:15 https://dx.doi.org/10.20517/2574-1225.2023.137 Page 5 of 7
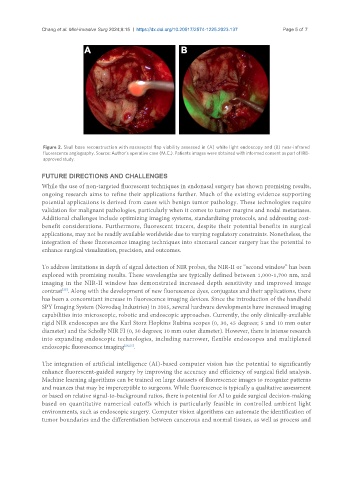
Figure 2. Skull base reconstruction with nasoseptal flap viability assessed in (A) white light endoscopy and (B) near-infrared
fluorescence angiography. Source: Author’s operative case (M.C.). Patients images were obtained with informed consent as part of IRB-
approved study.
FUTURE DIRECTIONS AND CHALLENGES
While the use of non-targeted fluorescent techniques in endonasal surgery has shown promising results,
ongoing research aims to refine their applications further. Much of the existing evidence supporting
potential applications is derived from cases with benign tumor pathology. These technologies require
validation for malignant pathologies, particularly when it comes to tumor margins and nodal metastases.
Additional challenges include optimizing imaging systems, standardizing protocols, and addressing cost-
benefit considerations. Furthermore, fluorescent tracers, despite their potential benefits in surgical
applications, may not be readily available worldwide due to varying regulatory constraints. Nonetheless, the
integration of these fluorescence imaging techniques into sinonasal cancer surgery has the potential to
enhance surgical visualization, precision, and outcomes.
To address limitations in depth of signal detection of NIR probes, the NIR-II or “second window” has been
explored with promising results. These wavelengths are typically defined between 1,000-1,700 nm, and
imaging in the NIR-II window has demonstrated increased depth sensitivity and improved image
contrast . Along with the development of new fluorescence dyes, conjugates and their applications, there
[25]
has been a concomitant increase in fluorescence imaging devices. Since the introduction of the handheld
SPY Imaging System (Novodaq Industries) in 2005, several hardware developments have increased imaging
capabilities into microscopic, robotic and endoscopic approaches. Currently, the only clinically-available
rigid NIR endoscopes are the Karl Storz Hopkins Rubina scopes (0, 30, 45 degrees; 5 and 10 mm outer
diameter) and the Scholly NIR FI (0, 30 degrees; 10 mm outer diameter). However, there is intense research
into expanding endoscopic technologies, including narrower, flexible endoscopes and multiplexed
endoscopic fluorescence imaging [26,27] .
The integration of artificial intelligence (AI)-based computer vision has the potential to significantly
enhance fluorescent-guided surgery by improving the accuracy and efficiency of surgical field analysis.
Machine learning algorithms can be trained on large datasets of fluorescence images to recognize patterns
and nuances that may be imperceptible to surgeons. While fluorescence is typically a qualitative assessment
or based on relative signal-to-background ratios, there is potential for AI to guide surgical decision-making
based on quantitative numerical cutoffs which is particularly feasible in controlled ambient light
environments, such as endoscopic surgery. Computer vision algorithms can automate the identification of
tumor boundaries and the differentiation between cancerous and normal tissues, as well as process and