Page 66 - Read Online
P. 66
Hambright et al. Mini-invasive Surg 2024;8:19 https://dx.doi.org/10.20517/2574-1225.2024.08 Page 9 of 12
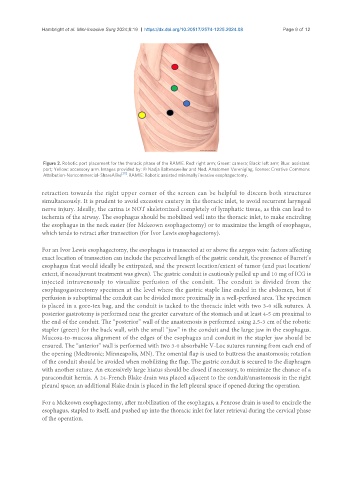
Figure 2. Robotic port placement for the thoracic phase of the RAMIE. Red: right arm; Green: camera; Black: left arm; Blue: assistant
port; Yellow: accessory arm. Images provided by: © Nadja Baltensweiler and Ned. Anatomen Vereniging, license: Creative Commons
[35]
Attribution-Noncommercial-ShareAlike . RAMIE: Robotic assisted minimally invasive esophagectomy.
retraction towards the right upper corner of the screen can be helpful to discern both structures
simultaneously. It is prudent to avoid excessive cautery in the thoracic inlet, to avoid recurrent laryngeal
nerve injury. Ideally, the carina is NOT skeletonized completely of lymphatic tissue, as this can lead to
ischemia of the airway. The esophagus should be mobilized well into the thoracic inlet, to make encircling
the esophagus in the neck easier (for Mckeown esophagectomy) or to maximize the length of esophagus,
which tends to retract after transection (for Ivor Lewis esophagectomy).
For an Ivor Lewis esophagectomy, the esophagus is transected at or above the azygos vein: factors affecting
exact location of transection can include the perceived length of the gastric conduit, the presence of Barrett’s
esophagus that would ideally be extirpated, and the present location/extent of tumor (and past location/
extent, if neoadjuvant treatment was given). The gastric conduit is cautiously pulled up and 10 mg of ICG is
injected intravenously to visualize perfusion of the conduit. The conduit is divided from the
esophagogastrectomy specimen at the level where the gastric staple line ended in the abdomen, but if
perfusion is suboptimal the conduit can be divided more proximally in a well-perfused area. The specimen
is placed in a gore-tex bag, and the conduit is tacked to the thoracic inlet with two 3-0 silk sutures. A
posterior gastrotomy is performed near the greater curvature of the stomach and at least 4-5 cm proximal to
the end of the conduit. The “posterior” wall of the anastomosis is performed using 2.5-3 cm of the robotic
stapler (green) for the back wall, with the small “jaw” in the conduit and the large jaw in the esophagus.
Mucosa-to-mucosa alignment of the edges of the esophagus and conduit in the stapler jaw should be
ensured. The “anterior” wall is performed with two 3-0 absorbable V-Loc sutures running from each end of
the opening (Medtronic; Minneapolis, MN). The omental flap is used to buttress the anastomosis; rotation
of the conduit should be avoided when mobilizing the flap. The gastric conduit is secured to the diaphragm
with another suture. An excessively large hiatus should be closed if necessary, to minimize the chance of a
paraconduit hernia. A 24-French Blake drain was placed adjacent to the conduit/anastomosis in the right
pleural space; an additional Blake drain is placed in the left pleural space if opened during the operation.
For a Mckeown esophagectomy, after mobilization of the esophagus, a Penrose drain is used to encircle the
esophagus, stapled to itself, and pushed up into the thoracic inlet for later retrieval during the cervical phase
of the operation.