Page 26 - Read Online
P. 26
Page 297 Berber et al. J Transl Genet Genom 2021;5:292-303 https://dx.doi.org/10.20517/jtgg.2021.35
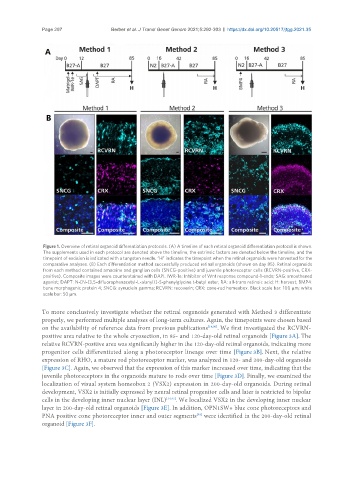
Figure 1. Overview of retinal organoid differentiation protocols. (A) A timeline of each retinal organoid differentiation protocol is shown.
The supplements used in each protocol are denoted above the timeline, the extrinsic factors are denoted below the timeline, and the
timepoint of excision is indicated with a tungsten needle. “H” indicates the timepoint when the retinal organoids were harvested for the
comparative analyses. (B) Each differentiation method successfully produced retinal organoids (shown on day 85). Retinal organoids
from each method contained amacrine and ganglion cells (SNCG-positive) and juvenile photoreceptor cells (RCVRN-positive, CRX-
positive). Composite images were counterstained with DAPI. IWR-1e: Inhibitor of Wnt response compound-1-endo; SAG: smoothened
agonist; DAPT: N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester; RA: all-trans retinoic acid; H: harvest; BMP4:
bone morphogenic protein 4; SNCG: synuclein gamma; RCVRN: recoverin; CRX: cone-rod homeobox. Black scale bar: 100 µm; white
scale bar: 50 µm.
To more conclusively investigate whether the retinal organoids generated with Method 3 differentiate
properly, we performed multiple analyses of long-term cultures. Again, the timepoints were chosen based
on the availability of reference data from previous publications [14,30] . We first investigated the RCVRN-
positive area relative to the whole cryosection, in 85- and 120-day-old retinal organoids [Figure 3A]. The
relative RCVRN-positive area was significantly higher in the 120-day-old retinal organoids, indicating more
progenitor cells differentiated along a photoreceptor lineage over time [Figure 3B]. Next, the relative
expression of RHO, a mature rod photoreceptor marker, was analyzed in 120- and 200-day-old organoids
[Figure 3C]. Again, we observed that the expression of this marker increased over time, indicating that the
juvenile photoreceptors in the organoids mature to rods over time [Figure 3D]. Finally, we examined the
localization of visual system homeobox 2 (VSX2) expression in 200-day-old organoids. During retinal
development, VSX2 is initially expressed by neural retinal progenitor cells and later is restricted to bipolar
cells in the developing inner nuclear layer (INL) [14,47] . We localized VSX2 in the developing inner nuclear
layer in 200-day-old retinal organoids [Figure 3E]. In addition, OPN1SW+ blue cone photoreceptors and
PNA positive cone photoreceptor inner and outer segments were identified in the 200-day-old retinal
[48]
organoid [Figure 3F].