Page 14 - Read Online
P. 14
Page 8 Bax. J Transl Genet Genom 2020;4:1-16 I http://dx.doi.org/10.20517/jtgg.2020.08
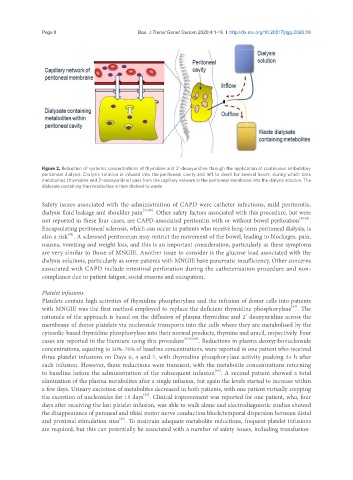
Figure 2. Reduction of systemic concentrations of thymidine and 2’-deoxyuridine through the application of continuous ambulatory
peritoneal dialysis. Dialysis solution is infused into the peritoneal cavity and left to dwell for several hours, during which time
metabolites (thymidine and 2’-deoxyuridine) pass from the capillary network in the peritoneal membrane into the dialysis solution. The
dialysate containing the metabolites is then drained to waste
Safety issues associated with the administration of CAPD were catheter infections, mild peritonitis,
dialysis fluid leakage and shoulder pain [74,89] . Other safety factors associated with this procedure, but were
not reported in these four cases, are CAPD-associated peritonitis with or without bowel perforation [90,91] .
Encapsulating peritoneal sclerosis, which can occur in patients who receive long-term peritoneal dialysis, is
[92]
also a risk . A sclerosed peritoneum may restrict the movement of the bowel, leading to blockages, pain,
nausea, vomiting and weight loss, and this is an important consideration, particularly as these symptoms
are very similar to those of MNGIE. Another issue to consider is the glucose load associated with the
dialysis solutions, particularly as some patients with MNGIE have pancreatic insufficiency. Other concerns
associated with CAPD include intestinal perforation during the catheterisation procedure and non-
compliance due to patient fatigue, social reasons and occupation.
Platelet infusions
Platelets contain high activities of thymidine phosphorylase and the infusion of donor cells into patients
[93]
with MNGIE was the first method employed to replace the deficient thymidine phosphorylase . The
rationale of the approach is based on the diffusion of plasma thymidine and 2’-deoxyuridine across the
membrane of donor platelets via nucleoside transports into the cells where they are metabolised by the
cytosolic-based thymidine phosphorylase into their normal products, thymine and uracil, respectively. Four
cases are reported in the literature using this procedure [87,93,94] . Reductions in plasma deoxyribonucleoside
concentrations, equating to 50%-70% of baseline concentrations, were reported in one patient who received
three platelet infusions on Days 0, 4 and 7, with thymidine phosphorylase activity peaking 24 h after
each infusion. However, these reductions were transient, with the metabolite concentrations returning
[93]
to baseline before the administration of the subsequent infusion . A second patient showed a total
elimination of the plasma metabolites after a single infusion, but again the levels started to increase within
a few days. Urinary excretion of metabolites decreased in both patients, with one patient virtually stopping
[93]
the excretion of nucleosides for 15 days . Clinical improvement was reported for one patient, who, four
days after receiving the last platelet infusion, was able to walk alone and electrodiagnostic studies showed
the disappearance of peroneal and tibial motor nerve conduction block/temporal dispersion between distal
[87]
and proximal stimulation sites . To maintain adequate metabolite reductions, frequent platelet infusions
are required, but this can potentially be associated with a number of safety issues, including transfusion-