Page 9 - Read Online
P. 9
Bax. J Transl Genet Genom 2020;4:1-16 I http://dx.doi.org/10.20517/jtgg.2020.08 Page 3
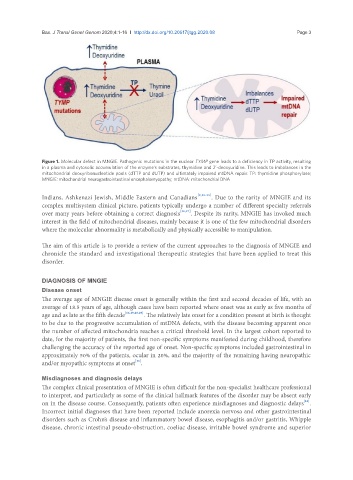
Figure 1. Molecular defect in MNGIE. Pathogenic mutations in the nuclear TYMP gene leads to a deficiency in TP activity, resulting
in a plasma and cytosolic accumulation of the enzyme’s substrates, thymidine and 2’-deoxyuridine. This leads to imbalances in the
mitochondrial deoxyribonucleotide pools (dTTP and dUTP) and ultimately impaired mtDNA repair. TP: thymidine phosphorylase;
MNGIE: mitochondrial neurogastrointestinal encephalomyopathy; mtDNA: mitochondrial DNA
Indians, Ashkenazi Jewish, Middle Eastern and Canadians [2,18-25] . Due to the rarity of MNGIE and its
complex multisystem clinical picture, patients typically undergo a number of different specialty referrals
over many years before obtaining a correct diagnosis [26,27] . Despite its rarity, MNGIE has invoked much
interest in the field of mitochondrial diseases, mainly because it is one of the few mitochondrial disorders
where the molecular abnormality is metabolically and physically accessible to manipulation.
The aim of this article is to provide a review of the current approaches to the diagnosis of MNGIE and
chronicle the standard and investigational therapeutic strategies that have been applied to treat this
disorder.
DIAGNOSIS OF MNGIE
Disease onset
The average age of MNGIE disease onset is generally within the first and second decades of life, with an
average of 18.5 years of age, although cases have been reported where onset was as early as five months of
age and as late as the fifth decade [16,19,28,29] . The relatively late onset for a condition present at birth is thought
to be due to the progressive accumulation of mtDNA defects, with the disease becoming apparent once
the number of affected mitochondria reaches a critical threshold level. In the largest cohort reported to
date, for the majority of patients, the first non-specific symptoms manifested during childhood, therefore
challenging the accuracy of the reported age of onset. Non-specific symptoms included gastrointestinal in
approximately 50% of the patients, ocular in 20%, and the majority of the remaining having neuropathic
[16]
and/or myopathic symptoms at onset .
Misdiagnoses and diagnosis delays
The complex clinical presentation of MNGIE is often difficult for the non-specialist healthcare professional
to interpret, and particularly as some of the clinical hallmark features of the disorder may be absent early
[16]
on in the disease course. Consequently, patients often experience misdiagnoses and diagnostic delays .
Incorrect initial diagnoses that have been reported include anorexia nervosa and other gastrointestinal
disorders such as Crohn’s disease and inflammatory bowel disease, esophagitis and/or gastritis, Whipple
disease, chronic intestinal pseudo-obstruction, coeliac disease, irritable bowel syndrome and superior