Page 15 - Read Online
P. 15
Bax. J Transl Genet Genom 2020;4:1-16 I http://dx.doi.org/10.20517/jtgg.2020.08 Page 9
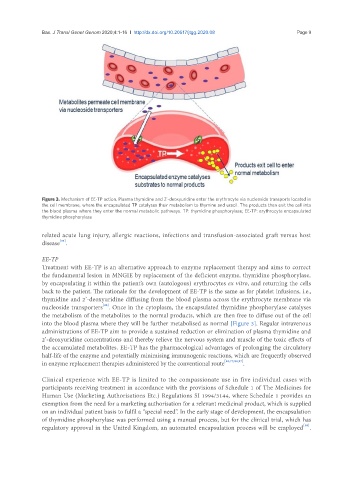
Figure 3. Mechanism of EE-TP action. Plasma thymidine and 2’-deoxyuridine enter the erythrocyte via nucleoside transports located in
the cell membrane, where the encapsulated TP catalyses their metabolism to thymine and uracil. The products then exit the cell into
the blood plasma where they enter the normal metabolic pathways. TP: thymidine phosphorylase; EE-TP: erythrocyte encapsulated
thymidine phosphorylase
related acute lung injury, allergic reactions, infections and transfusion-associated graft versus host
[95]
disease .
EE-TP
Treatment with EE-TP is an alternative approach to enzyme replacement therapy and aims to correct
the fundamental lesion in MNGIE by replacement of the deficient enzyme, thymidine phosphorylase,
by encapsulating it within the patient’s own (autologous) erythrocytes ex vitro, and returning the cells
back to the patient. The rationale for the development of EE-TP is the same as for platelet infusions, i.e.,
thymidine and 2’-deoxyuridine diffusing from the blood plasma across the erythrocyte membrane via
[96]
nucleoside transporters . Once in the cytoplasm, the encapsulated thymidine phosphorylase catalyses
the metabolism of the metabolites to the normal products, which are then free to diffuse out of the cell
into the blood plasma where they will be further metabolised as normal [Figure 3]. Regular intravenous
administrations of EE-TP aim to provide a sustained reduction or elimination of plasma thymidine and
2’-deoxyuridine concentrations and thereby relieve the nervous system and muscle of the toxic effects of
the accumulated metabolites. EE-TP has the pharmacological advantages of prolonging the circulatory
half-life of the enzyme and potentially minimising immunogenic reactions, which are frequently observed
in enzyme replacement therapies administered by the conventional route [48,77,84,97] .
Clinical experience with EE-TP is limited to the compassionate use in five individual cases with
participants receiving treatment in accordance with the provisions of Schedule 1 of The Medicines for
Human Use (Marketing Authorisations Etc.) Regulations SI 1994/3144, where Schedule 1 provides an
exemption from the need for a marketing authorisation for a relevant medicinal product, which is supplied
on an individual patient basis to fulfil a “special need”. In the early stage of development, the encapsulation
of thymidine phosphorylase was performed using a manual process, but for the clinical trial, which has
[98]
regulatory approval in the United Kingdom, an automated encapsulation process will be employed .