Page 31 - Read Online
P. 31
Brault et al. J Transl Genet Genom. 2025;9:1-10 https://dx.doi.org/10.20517/jtgg.2024.83 Page 5
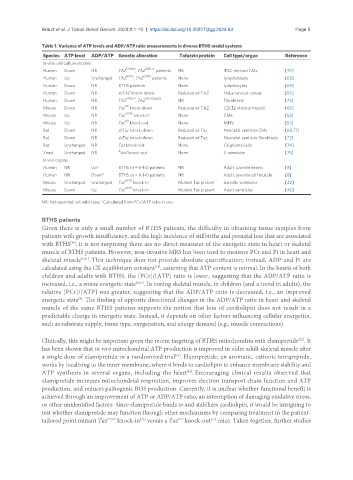
Table 1. Variance of ATP levels and ADP/ATP ratio measurements in diverse BTHS model systems
Species ATP level ADP/ATP Genetic alteration Tafazzin protein Cell type/organ Reference
In vitro cell culture models
517delG 328T>C
Human Down NR TAZ , TAZ patients NR iPSC-derived CMs [70]
G197E I209D
Human Up Unchanged TAZ , TAZ patients None lymphoblasts [68]
Human Down NR BTHS patients None lymphocytes [69]
Human Down NR shTAZ knock-down Reduced wt TAZ HeLa cervical cancer [50]
Human Down NR TAZ 170G>T , TAZ 140-152del13 NR Fibroblasts [73]
KD
Mouse Down NR Taz knock-down Reduced wt TAZ C2C12 skeletal muscle [60]
Mouse Up NR Taz G193V knock-in None CMs [53]
KO
Mouse Up NR Taz knock-out None MEFs [53]
Rat Down NR shTaz knock-down Reduced wt Taz Neonatal ventricle CMs [60,71]
Rat Down NR shTaz knock-down Reduced wt Taz Neonatal ventricle fibroblasts [72]
Rat Unchanged NR Taz knock-out None C6 glioma cells [74]
Δ
Yeast Unchanged NR taz1 knock-out None S. cerevisiae [75]
In vivo organs
Human NR Up* BTHS (n = 6-14) patients NR Adult, juvenile hearts [8]
Human NR Down* BTHS (n = 6-14) patients NR Adult, juvenile calf muscle [8]
Mouse Unchanged Unchanged Taz D57H knock-in Mutant Taz present Juvenile ventricles [42]
D57H
Mouse Down Up Taz knock-in Mutant Taz present Adult ventricles [42]
NR: Not reported; wt: wild-type; *Calculated from PCr/ATP ratio in vivo.
BTHS patients
Given there is only a small number of BTHS patients, the difficulty in obtaining tissue samples from
patients with growth insufficiency, and the high incidence of stillbirths and prenatal loss that are associated
with BTHS , it is not surprising there are no direct measures of the energetic state in heart or skeletal
[80]
muscle of BTHS patients. However, non-invasive MRS has been used to measure PCr and Pi in heart and
skeletal muscle [8,81] . This technique does not provide absolute quantification; instead, ADP and Pi are
calculated using the CK equilibrium constant , assuming that ATP content is normal. In the hearts of both
[14]
children and adults with BTHS, the [PCr]/[ATP] ratio is lower, suggesting that the ADP/ATP ratio is
increased, i.e., a worse energetic state [8,81] . In resting skeletal muscle, in children (and a trend in adults), the
relative [PCr]/[ATP] was greater, suggesting that the ADP/ATP ratio is decreased, i.e., an improved
energetic state . The finding of opposite directional changes in the ADP/ATP ratio in heart and skeletal
[8]
muscle of the same BTHS patients supports the notion that loss of cardiolipin does not result in a
predictable change in energetic state. Instead, it depends on other factors influencing cellular energetics,
such as substrate supply, tissue type, oxygenation, and energy demand (e.g., muscle contractions).
[82]
Clinically, this might be important given the recent targeting of BTHS mitochondria with elamipretide . It
has been shown that in vivo mitochondrial ATP production is improved in older adult skeletal muscle after
a single dose of elamipretide in a randomized trial . Elamipretide, an aromatic, cationic tetrapeptide,
[83]
works by localizing to the inner membrane, where it binds to cardiolipin to enhance membrane stability and
ATP synthesis in several organs, including the heart . Encouraging clinical results observed that
[84]
elamipretide increases mitochondrial respiration, improves electron transport chain function and ATP
production, and reduces pathogenic ROS production. Currently, it is unclear whether functional benefit is
achieved through an improvement of ATP or ADP/ATP ratio, an interruption of damaging oxidative stress,
or other unidentified factors. Since elamipretide binds to and stabilizes cardiolipin, it would be intriguing to
test whether elamipretide may function through other mechanisms by comparing treatment in the patient-
tailored point mutant Taz D75H knock-in versus a Taz knock-out mice. Taken together, further studies
[77]
KO
[42]