Page 88 - Read Online
P. 88
Thomas et al. J Transl Genet Genom 2024;8:249-77 https://dx.doi.org/10.20517/jtgg.2024.15 Page 265
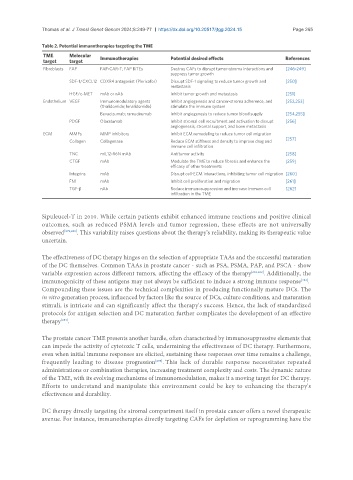
Table 2. Potential immunotherapies targeting the TME
TME Molecular Immunotherapies Potential desired effects References
target target
Fibroblasts FAP FAP-CAR-T; FAP BiTEs Destroy CAFs to disrupt tumor-stroma interactions and [246-249]
suppress tumor growth
SDF-1/CXCL12 CDXR4 antagonist (Plerixafor) Disrupt SDF-1 signaling to reduce tumor growth and [250]
metastasis
HGF/c-MET mAb or nAb Inhibit tumor growth and metastasis [251]
Endothelium VEGF Immunomodulatory agents Inhibit angiogenesis and cancer-stroma adherence, and [252,253]
(thalidomide; lenalidomide) stimulate the immune system
Bevacizumab; ramucirumab Inhibit angiogenesis to reduce tumor blood supply [254,255]
PDGF Olaratumab Inhibit stromal cell recruitment and activation to disrupt [256]
angiogenesis, stromal support, and bone metastasis
ECM MMPs MMP inhibitors Inhibit ECM remodeling to reduce tumor cell migration
[257]
Collagen Collagenase Reduce ECM stiffness and density to improve drug and
immune cell infiltration
TNC mIL12-R6N mAb Antitumor activity [258]
CTGF mAb Modulate the TME to reduce fibrosis and enhance the [259]
efficacy of other treatments
Integrins mAb Disrupt cell-ECM interactions, inhibiting tumor cell migration [260]
FN1 mAb Inhibit cell proliferation and migration [261]
TGF-β nAb Reduce immunosuppression and increase immune cell [262]
infiltration in the TME
Sipuleucel-T in 2010. While certain patients exhibit enhanced immune reactions and positive clinical
outcomes, such as reduced PSMA levels and tumor regression, these effects are not universally
observed [279,280] . This variability raises questions about the therapy's reliability, making its therapeutic value
uncertain.
The effectiveness of DC therapy hinges on the selection of appropriate TAAs and the successful maturation
of the DC themselves. Common TAAs in prostate cancer - such as PSA, PSMA, PAP, and PSCA - show
variable expression across different tumors, affecting the efficacy of the therapy [281,282] . Additionally, the
immunogenicity of these antigens may not always be sufficient to induce a strong immune response .
[283]
Compounding these issues are the technical complexities in producing functionally mature DCs. The
in vitro generation process, influenced by factors like the source of DCs, culture conditions, and maturation
stimuli, is intricate and can significantly affect the therapy's success. Hence, the lack of standardized
protocols for antigen selection and DC maturation further complicates the development of an effective
therapy .
[284]
The prostate cancer TME presents another hurdle, often characterized by immunosuppressive elements that
can impede the activity of cytotoxic T cells, undermining the effectiveness of DC therapy. Furthermore,
even when initial immune responses are elicited, sustaining these responses over time remains a challenge,
frequently leading to disease progression . This lack of durable response necessitates repeated
[285]
administrations or combination therapies, increasing treatment complexity and costs. The dynamic nature
of the TME, with its evolving mechanisms of immunomodulation, makes it a moving target for DC therapy.
Efforts to understand and manipulate this environment could be key to enhancing the therapy's
effectiveness and durability.
DC therapy directly targeting the stromal compartment itself in prostate cancer offers a novel therapeutic
avenue. For instance, immunotherapies directly targeting CAFs for depletion or reprogramming have the